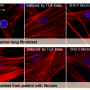
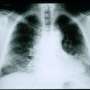
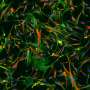
Fibrosis describes the accumulation of excessive of connective tissue that occurs in response to organ injury or pathological states. Scars that replace injured skin are an example of external, visible fibrosis. Progressive scarring of the lung, liver, and heart represent fibrotic diseases that are major drivers of mortality. The tissue stiffness that characterizes fibrosis is also known to play a role in cancer metastasis.
Fibrosis-initiating cells, or fibroblasts, respond to signaling by the growth factor TGF-β, but this molecule also acts on epithelial and immune cells to suppress inflammation and autoimmunity. Global and chronic inhibition of TGF-β signaling has numerous adverse effects, necessitating a more specific approach to blocking fibrosis-initiating TGF-β pathways. Using a high-throughout screen of small molecules, researchers in Harold Chapman's lab at UCSF School of Medicine identified a class of compounds called trihydroxyphenolics as inhibitors of TGF-β1-induced responses in fibroblasts. They observed that trihydroxyphenolics do not affect non-fibrogenic epithelial or immune cells. Further studies determined that fibroblasts and fibroblast-like cancer cells produce a specific protein that generates an intermediate metabolite of trihydroxyphenolic compounds. As only fibrosis-initiating cells produce the metabolite, trihydroxyphenol treatment selectively affects fibrogenic cells.
As the group describes in a study published this week in the JCI, the identification of this class of compounds as TGF-β inhibitors with selective anti-fibrotic activity delineates a possible therapeutic approach to attenuate fibrosis without the harmful side effects of non-specific TGF-β inhibition.
More information: Ying Wei et al, Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis, Journal of Clinical Investigation (2017). DOI: 10.1172/JCI94624
Journal information: Journal of Clinical Investigation
Provided by JCI