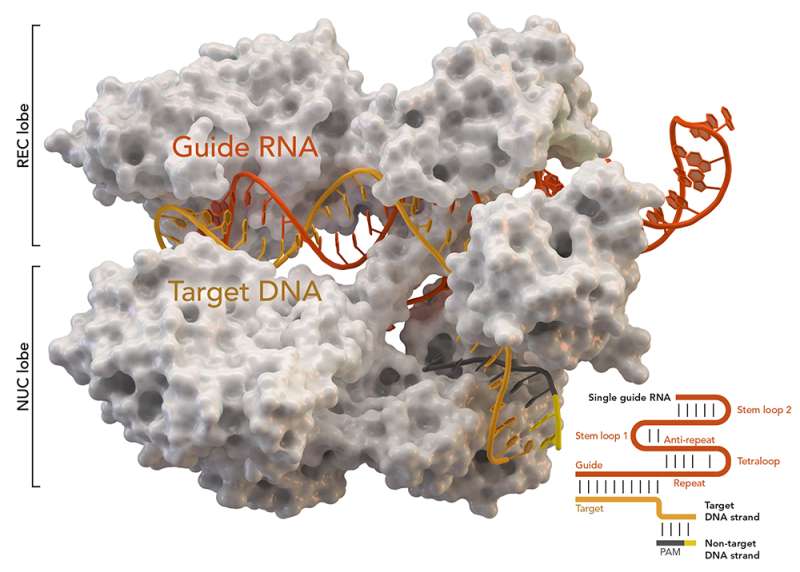
CRISPR-associated protein Cas9 (white) from Staphylococcus aureus based on Protein Database ID 5AXW. Credit: Thomas Splettstoesser (Wikipedia, CC BY-SA 4.0)
In preclinical trials, Stanford scientists and their collaborators harnessed the gene-editing system CRISPR-Cas9 to replace the mutated gene underpinning the devastating immune disease.
Very rarely, a boy is born with a mutation that renders his immune system barren—devoid of any and all immune cells. The disease, X-linked severe combined immunodeficiency, or SCID-X1, often is referred to as the bubble boy disease. It affects only males and is lethal if not treated in the first year of life.
Now, scientists at the School of Medicine and their collaborators have used the gene-editing system CRISPR-Cas9 to devise a new treatment to replenish immune cells in mouse models of SCID-X1. The results are promising, the scientists said, because they believe the treatment could potentially work in humans, as well.
SCID-X1 affects about 1 in 50,000 male births. Those with the disease suffer from a debilitating mutation in a single gene, IL2R gamma. When this gene is defective, the immune system never develops.
The standard treatment for patients with SCID-X1 is a bone marrow transplant, which supplies them with stem cells that will give rise to a working immune system. But the transfer process is tricky and not guaranteed to work. So, Matthew Porteus, MD, Ph.D., professor of pediatrics, came up with a new idea: correct the genes in the patients' own cells.
Through CRISPR-Cas9, Porteus and his team have done just that. Using cell samples that came from people with SCID-X1, the researchers genetically altered the class of stem cells that give rise to blood and immune cells. Their approach got the gene working again.
Each mouse that received the edited cells began generating new immune cells and displayed no detectable adverse side effects. "To our knowledge, it's the first time that human SCID-X1 cells edited with CRISPR-Cas9 have been successfully used to make human immune cells in an animal model," said postdoctoral scholar Mara Pavel-Dinu, Ph.D.
A paper describing the work was published online April 9 in Nature Communications. Porteus is the senior author, and Pavel-Dinu is the first author.
Editing in a solution
Gene-based therapy for SCID is not new. In the 1990s, scientists began to dabble in gene therapies that used a virus to deliver a new, functional IL2R gamma gene. "It was very effective, but about 25 percent of the patients developed a leukemia because the virus integrated into an erroneous gene," Porteus said. "It showed both the promise of what gene therapy could do and highlighted the area that needed to be improved."
Porteus' approach uses CRISPR-Cas9 to create a double-stranded break in DNA to insert a healthy copy of the IL2R gamma gene in the stem cells that create immune cells.
Using the gene-editing system, scientists tweaked cells from six people with SCID-X1 and then transplanted those cells into mouse models of SCID-X1. Those mice were then not only able to make their own immune cells, but many of the edited cells retained something called "stemness," meaning that they maintained their ability to continually create new cells.
"The idea is that these modified stem cells will give rise to the blood system and the immune system for the entirety of the patient's life, which we hope is 90 or more years," Porteus said. "And we see evidence for that in our study."
Popping the bubble
"We've showed that this is a novel and effective strategy to potentially treat this disease, but the other big thing here is safety," Porteus said. "We don't see any abnormalities in the mice that receive the treatment. More specifically, we also performed genetic analysis to see if the CRISPR-Cas9 system made DNA breaks at places that it's not supposed to, and we see no evidence of that." That's crucial, Porteus said, because it ensures that other healthy genes aren't being erroneously tampered with.
Translating lab research to a patient population takes time, Porteus said, but he's optimistic that if larger mouse studies are successful, the CRISPR-Cas9 gene therapy could be piloted in human patients in the next year or two through the Stanford Center for Definitive and Curative Medicine.
More information: Mara Pavel-Dinu et al. Gene correction for SCID-X1 in long-term hematopoietic stem cells, Nature Communications (2019). DOI: 10.1038/s41467-019-09614-y
Journal information: Nature Communications
Provided by Stanford University Medical Center