This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists track earliest cancer-triggering physical changes in cells
By the time cancer is diagnosed, a lot has already happened behind the scenes. Although cancers are classed into early and late stages for clinical purposes, even an "early" stage tumor is the result of many previous, undetectable cellular and molecular changes in the body.
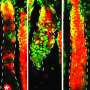
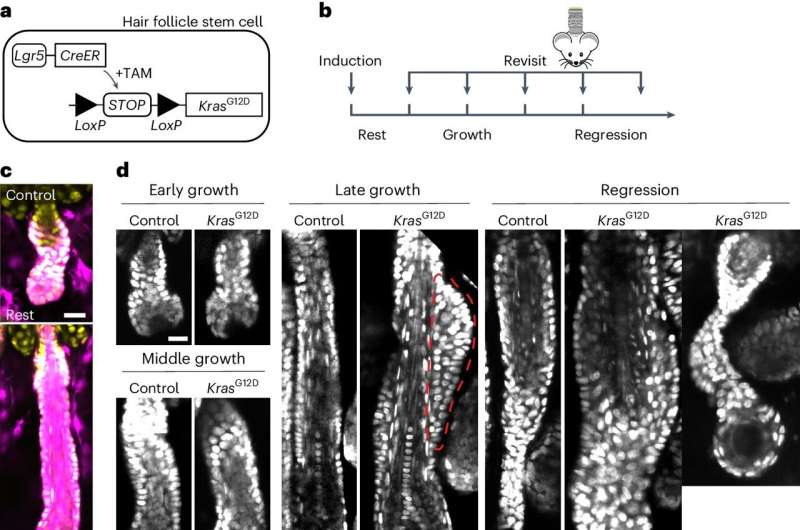
Now, scientists at Yale School of Medicine (YSM) and their collaborators have caught a detailed glimpse of some of those earliest changes, using powerful, high-resolution microscopy to track the very first cancer-triggering physical changes in mouse skin cells.
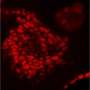
By studying mice that carry a cancer-promoting mutation in their hair follicles, the scientists found that the earliest signs of cancer formation happen at a precise time and place in the growth of the hair follicles of the mouse skin cells. Further, they found that those precancerous changes can be blocked with a kind of drug known as an MEK inhibitor.
The team was led by Tianchi Xin, Ph.D., research scientist in genetics at YSM, and included Valentina Greco, Ph.D., Carolyn Walch Slayman Professor of Genetics at YSM and member of the Yale Cancer Center and Yale Stem Cell Center, and Sergi Regot, Ph.D., associate professor of molecular biology and genetics at Johns Hopkins School of Medicine.
They published the results of their research on April 30 in the journal Nature Cell Biology.
The scientists studied mice that develop cutaneous squamous cell carcinoma, the second most common type of human skin cancer. These mice were genetically engineered with a cancer-promoting mutation in a gene called KRAS, which is among the most commonly mutated oncogenes in human cancers. KRAS mutations have also been found to drive lung cancer, pancreatic cancer, and colorectal cancer, among others.
The early change that the scientists studied—the growth of a tiny, abnormal bump in the hair follicle—is classified as a pre-cancerous abnormality. "Understanding these early events could be helpful for us to actually design approaches to prevent the eventual formation of cancer," said Xin, who was first author on the study.
Although their study focused on skin cancer, the researchers believe the principles they discovered are likely to apply to the many other kinds of cancer driven by KRAS mutations because the basic genes and proteins involved are the same across different tumors.
Not just cell proliferation
As in humans, mouse hair follicles continually grow, shedding old hairs and forming new ones. Stem cells, which hold the capacity to develop into many other kinds of cells, drive much of this renewal cycle. Previous studies had found that KRAS mutations drive an increase in stem cell proliferation in hair follicles, and it was assumed this significant increase in the number of stem cells was responsible for the precancerous tissue disruption.
To test this assumption, the team used a specially engineered form of mutated KRAS that they could switch on at a specific time in the animals' hair follicle skin cells. Xin and his colleagues used a microscopy method known as intravital imaging, whose name refers to the technique's ability to image cells at high resolution in a living animal, and to label and follow individual stem cells in the animals.
When the KRAS mutation was triggered, all the stem cells proliferated faster, but the pre-cancerous bump only formed in one specific place in the hair follicle and in one stage in the growth cycle, meaning that the overall higher number of cells was likely not the whole story.
Switching on the KRAS mutation in hair follicles led the stem cells to proliferate faster, change their migration patterns, and divide in different directions from cells without the cancer-promoting mutation.
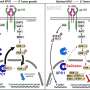
The mutation acts on a downstream protein known as ERK. Xin was able to observe real-time ERK activity in individual stem cells in live animals and discovered a specific change in that protein's activity triggered by the KRAS mutation. The researchers were also able to stop the formation of the pre-cancerous bump using an MEK inhibitor, which blocks ERK's activity.
The drug halted the mutation's effects on migration and cell orientation, but not on overall stem cell proliferation, implying that formation of the pre-cancerous condition is due to these first two changes, rather than to enhanced cell proliferation.
Precancer in context
Tracking the effects of an oncogenic mutation in real time, in a living animal, is the only way the researchers were able to uncover these principles. That is important because cancer does not form in a vacuum—it is highly dependent on its microenvironment to grow and sustain itself. The scientists also needed to track not only the behavior of individual cells, but the molecules within those cells.
"The way we've approached understanding these oncogenic events is really to connect across scale," Greco said. "The framework and approaches Dr. Xin used in collaboration with Dr. Regot allowed us to go down to molecular elements while connecting them to the scale of the cell and the tissue, in a way that gives us a resolution to these events that's so difficult to do outside a living animal."
Next, the researchers want to track the process for a longer time to see what happens after that initial bump forms. They also want to look at other cancer-promoting events like inflammation to see if the principles they discovered apply in other contexts.
More information: Tianchi Xin et al, Oncogenic Kras induces spatiotemporally specific tissue deformation through converting pulsatile into sustained ERK activation, Nature Cell Biology (2024). DOI: 10.1038/s41556-024-01413-y