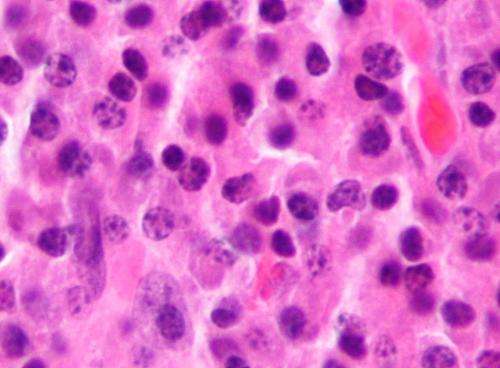
Micrograph of a plasmacytoma, the histologic correlate of multiple myeloma. H&E stain. Credit: Wikipedia/CC BY-SA 3.0
A first-in-class drug recently granted accelerated approval by the U.S. Food and Drug Administration (FDA) for adult patients with heavily pretreated multiple myeloma has been the subject of study at Moffitt Cancer Center for years. Now, selinexor (XPOVIO, Karyopharm Therapeutics) used in combination with the corticosteroid dexamethasone will offer another option for patients with multiple myeloma who have exhausted the most common therapies for the disease.
Unlike other cancer drugs, selinexor works as a selective inhibitor of nuclear export (SINE). SINE drugs gain access to cells and target the mechanisms that move molecules out of the cell nucleus. Specifically, SINE targets XPO1, a molecule that chaperones the nuclear export of more than 250 proteins. Among those exported by XPO1 are the tumor suppressor proteins (TSP), which suppress tumor growth. When selinexor is present, TSP accumulates in the cell nucleus, leading to cell death.
Moffitt researchers led by Associate Center Director of Clinical Science Daniel Sullivan, M.D. have been investigating the nuclear export of proteins in cancer cells for more than 12 years, and working with Karyopharm to investigate SINE agents for the past seven years. Using multiple myeloma cell lines and patient bone marrow aspirates, they helped to define how SINE molecules reduced cancer cell viability and induced cell death. They also demonstrated how SINE molecules used alone or in conjunction with chemotherapeutics could overcome acquired drug resistance in multiple myeloma.
"Specifically, we have found that selinexor synergistically interacts with doxorubicin, proteasome inhibitors, and melphalan—all drugs used in the treatment of multiple myeloma," said Sullivan. These pre-clinical findings from Sullivan's laboratory are the basis for two ongoing trials at Moffitt in multiple myeloma: a trial using selinexor in combination with doxorubicin and dexamethasone led by Rachid Baz, M.D., and one using selinexor plus high-dose melphalan led by Taiga Nishihori, M.D.
Moffitt was part of the multicenter STORM trial that led to selinexor's accelerated FDA approval. Baz, myeloma section head and director of clinical research in the Department of Malignant Hematology at Moffitt, co-authored the STORM study published in the Journal of Clinical Oncology in March 2018. The phase 2 trial evaluated efficacy of oral selinexor along with the corticosteroid dexamethasone in patients whose multiple myeloma was refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.
Analysis of a pre-specified subgroup of 83 patients whose disease was refractory to bortezomib, carfilzomib, lenalidomide, pomalidomide and daratumumab yielded an overall response rate of 25.3%. Median time to first response was 4 weeks, and median duration of response was 2.8 months.
"Selinexor, with a novel mechanism of action, represents another treatment option for patients with multiple myeloma," said Baz. "While the response rate in patients with advanced myeloma is modest, ongoing studies which are evaluating its role in combinations with other agents are promising."
Multiple myeloma (MM) is a cancer in plasma cells, a type of white blood cell that plays a key role in fighting infections. When plasma cells become cancerous and grow uncontrolled in the bone marrow, they interfere with normal blood production which can lead to anemia and increased infections. The plasma cells' unchecked growth may also result in multiple bone lesions that increase the risks of fracture. Multiple myeloma is the second most common blood cancer in the United States. The National Cancer Institute estimates 32,000 new cases will be diagnosed nationwide in 2019, with nearly 13,000 deaths due to MM anticipated this year.
Though MM patients can live years with their disease held in check by several anti-myeloma agents, almost all patients eventually will develop disease that is resistant to the most commonly used drugs. The prognosis for relapsed or refractory multiple myeloma (RRMM) is poor, highlighting the need for new therapeutic agents such as selinexor.
Moffitt researchers continue to study the possible expansion of selinexor's use in other malignancies, including advanced solid tumors, metastatic breast cancer, acute myeloid leukemia and relapsed/refractory non-Hodgkin lymphoma.
Journal information: Journal of Clinical Oncology