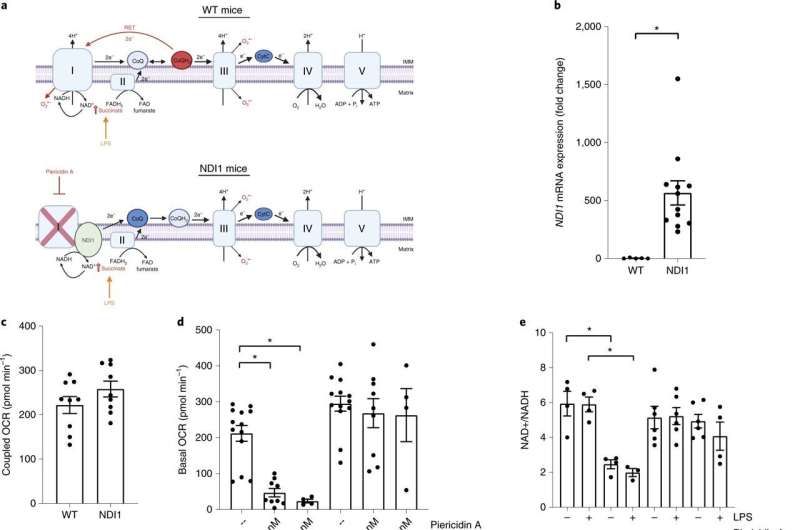
NDI1 expression confers resistance to mitochondrial complex I inhibitor piericidin A. a, Schematic of the mitochondrial electron transport chain in WT (top) and NDI1-expressing (bottom) BMDMs during LPS stimulation. Piericidin A inhibition of mitochondrial complex I on electron flow is rescued by NDI1 expression. IMM, inner mitochondrial membrane; RET, reverse electron transport. b, NDI1 mRNA levels (ΔΔCt) in WT and NDI1 BMDMs (n = 5 WT; n = 12 NDI1). c, Coupled OCR in WT and NDI1 BMDMs (n = 9 for each genotype). d, Basal OCR in WT and NDI1 BMDMs after 1 h treatment with 100 nM or 500 nM piericidin A (n = 13 vehicle for each genotype; n = 9 100 nM piericidin A for each genotype; n = 4 500 nM piericidin A for each genotype). e, NAD+/NADH ratio in WT and NDI1 BMDMs after 4 h treatment with or without LPS (100 ng ml–1) in the presence or absence of piericidin A (500 nM) (n = 3 WT LPS + piercidin A; n = 4 all other treatments). f, Rate of H2O2 production in WT and NDI1 BMDMs in the presence of succinate (500 μM) with or without piericidin A treatment (500 nM) (n = 9). g, Heatmap of significantly altered metabolites in WT and NDI1 BMDMs treated with LPS (100 ng ml–1) alone, piericidin A alone (500 nM) or both LPS and piericidin A for 4 h. The relative abundance of each metabolite is depicted as z score across rows (red, high; blue, low) (n = 5 for all treatments). h, Arbitrary units of succinate in WT and NDI1 BMDMs with or without LPS (100 ng ml–1) and piericidin A (500 nM) for 4 h (n = 5 for all treatments). Data are mean ± s.e.m. *P < 0.05, two-tailed t-test (b, P = 0.0001), ANOVA with Tukey’s post hoc test for multiple comparisons (d, *P = 0.0008 WT UT/100 nM, *P = 0.0047 WT UT/500 nM; e, *P = 0.006 WT UT/WT piericidin A, *P = 0.0034 WT LPS/WT LPS + piericidin A; f, *P = 0.0465 WT succinate/WT succinate + piericidin A, *P = 0.0493 NDI1 succinate/NDI1 succinate + piericidin A; h, *P = 0.0478), or ANOVA with Fisher’s LSD (g). n indicates number of individual mice. Parts of this figure were created with BioRender.com. Credit: Nature Immunology (2022). DOI: 10.1038/s41590-022-01185-3
Northwestern Medicine investigators recently discovered that the mitochondrial respiratory chain—a series of protein complexes essential for a cellular respiration and energy production—is necessary for the activation of another protein complex linked to inflammation and the progression of chronic diseases, according to a study published in Nature Immunology.
"Our study revealed that inhibition of mitochondrial respiratory chain dampened NLRP3 inflammasome activation genetically and pharmacologically," said Navdeep Chandel, Ph.D., the David W. Cugell, MD, Professor of Medicine in the Division of Pulmonary and Critical Care and of Biochemistry and Molecular Genetics, and senior author of the study.
The NLRP3 inflammasome is a protein complex that is activated by viral or bacterial infections and can also cause tissue damage and the progression of chronic disease, including obesity, Alzheimer's disease and type 2 diabetes, by triggering the release of proinflammatory cytokines.
Previous work had demonstrated that the NLRP3 inflammasome is regulated by mitochondria, specifically through the generation of mitochondrial reactive oxygen species (ROS), notably superoxide and hydrogen peroxide.
In the current study, Chandel and his team used genetic sequencing and pharmacologic strategies to study macrophages (specialized white blood cells) and examine the necessity of mitochondrial ROS for the activation of the NLRP3 inflammasome.
To their surprise, they discovered that mitochondrial ROS were not necessary. Furthermore, inhibitors of the mitochondrial respiratory chain complexes I, II, III and V—all of which are essential for proper production of energy within the cell—prevented NLRP3 inflammasome activation.
They also found that creatine, which is naturally produced by the body and is a widely available nutritional supplement, boosts levels of phosphocreatine, which is essential for proper energy production within cells, and is depleted by the respiratory chain inhibitors, which in turn also sustains activation of the inflammasome.
"The discovery that creatine and phosphocreatine are linked to inflammation opens a new avenue of research with possible implications for creatine," Chandel said.
According to the authors, the current findings highlight that the mitochondrial respiratory chain sustains NLRP3 inflammasome activation through phosphocreatine-dependent generation of energy and is completely independent of mitochondrial ROS.
Chandel said his team is now interested in investigating how mitochondria control inflammation in the contexts of influenza and Alzheimer's disease.
"The tools and conceptual framework we generated in this paper will allow us and others to examine how mitochondrial regulate variety of cytokines in distinct diseases," Chandel said.
More information: Leah K. Billingham et al, Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation, Nature Immunology (2022). DOI: 10.1038/s41590-022-01185-3
Journal information: Nature Immunology
Provided by Northwestern University