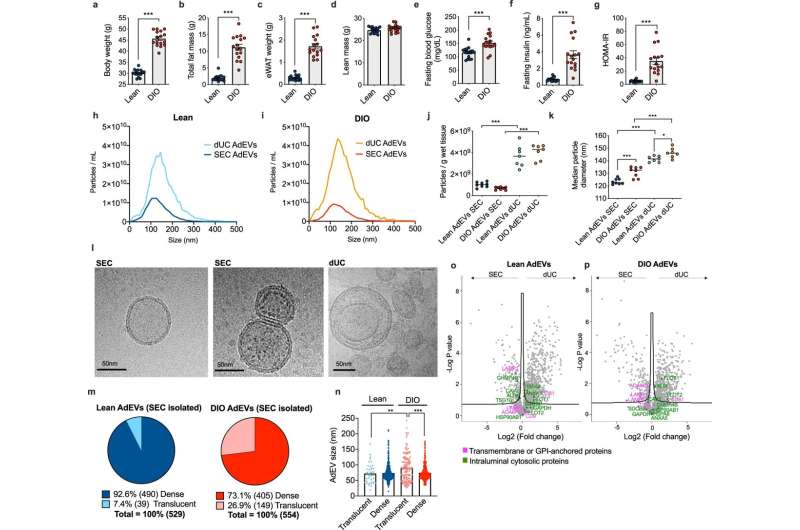
Comparative assessment of AdEVs isolated by dUC or SEC. Male lean and diet-induced obese (DIO) C57BL/6 J mice (n = 16 mice, 24–26 weeks of age, 16 weeks of feeding) were assessed for a body weight, b fat mass, c epididymal white adipose tissue (eWAT) mass, d lean mass, e fasting glucose, f fasting insulin, and g HOMA-IR values. h–k Nanoparticle Tracking Analysis (NTA) of adipocyte-derived extracellular vesicles (AdEVs, n = 7–8 biological replicates from two different EV isolation experiments) with size distributions of AdEVs from h lean and i DIO mice, j numbers of particles per gram wet tissue and k median particle diameters of the isolated EVs. l Representative cryo-TEM images from two independent AdEVs isolations using size exclusion chromatography (SEC) with translucent (left) and dense (middle) lumen or by differential ultracentrifugation (dUC) (right image). m Cryo-TEM-determined dense/translucent percentage, n median AdEV diameter of dense and translucent AdEVs. o–p LC-MS/MS analysis of the protein abundance of EV markers listed in the MISEV guideline. Comparison of EV markers in SEC- and dUC-isolated AdEVs from o lean and p DIO mice (n = 4 independent AdEV replicate samples. Each AdEV sample was isolated from visceral fat pads pooled from 5 DIO or 10 lean male 6–8 months old C57BL/6 J mice subjected to 4–6 months of HFD or chow). a–p Data are presented as mean values ± SEM, asterisks indicate *P < 0.05, **P < 0.01, ***P < 0.001. a–g Significance was determined by unpaired two-tailed t-tests, j, k, n One-Way ANOVA with Sidak’s post-test, or o, p One-Way ANOVA with a false discovery rate (FDR) of 0.1. Exact P values are (a–b, f, g, dietary effects) P < 0.0001, e P = 0.0003, (j isolation methods) P < 0.0001, k P < 0.0001 for all comparisons except for P = 0.0004 for lean AdEVs SEC vs. DIO AdEVs SEC and P = 0.390 for lean AdEVs dUC vs. DIO AdEVs dUC. Exact P values for EV size and lumina morphology are n P = 0.0011 (AdEV size of lean translucent vs. DIO translucent) and P < 0.0001 (AdEV size of DIO translucent vs. DIO dense). Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-36148-1
Scientists at the University of Augsburg and Helmholtz Munich have made an important breakthrough in better understanding early processes in the development of type 2 diabetes by identifying a previously unknown transmission of messenger substances from adipose tissue to the pancreas.
In a publication in Nature Communications, the team led by Prof. Dr. Kerstin Stemmer was able to show that adipose cells release tiny lipid membrane particles known as extracellular vesicles into the blood, which can stimulate the release of the blood sugar-lowering hormone insulin from the pancreas.
Adipose tissue has a bad reputation. This is not least because of the increasing number of overweight and obese people worldwide. Adipose tissue cells are highly efficient energy stores that convert excess calories from food into fat deposits, often of considerable size. Nevertheless, body fat is not generally bad as it has extremely important functions.
For example, as an endocrine, a hormone-producing organ, adipose tissue is involved in the regulation of many bodily processes. Researchers at the University of Augsburg and Helmholtz Munich, member of the German Center for Diabetes Research, have now succeeded in revealing another function of adipose tissue. This is because fat cells not only release hormones into the blood but also so-called extracellular vesicles.
"Extracellular vesicles are small membrane-enveloped particles that are released from all body cells and carry a kind of snapshot of cellular events through the body. They can be compared to Trojan horses that transport proteins, lipids, and nucleic acids to a target tissue for release. Once in the new cell, they can alter its function," explains Konxhe Kulaj, Ph.D. candidate and first author of the article.
"For example, extracellular vesicles from fat cells are targeted to the beta cells of the pancreas, where they are taken up and increase the release of the hormone insulin," Kulaj continues.
Healthy and obese adipose tissue on the move with different 'cargo'
Together with doctoral candidate Michaela Bauer, her colleague Dr. Alexandra Harger, and with the help of proteome researchers Dr. Natalie Krahmer and Özüm Sehnaz Caliskan from Helmholtz Munich, Kulaj was able to demonstrate in a series of experiments that extracellular vesicles from healthy and obese adipose tissue carry a very different composition of messenger substances as "cargo" and thus influence the function of beta cells of the pancreas in different ways.
If the extracellular vesicles originated from healthy adipose tissue, as is the case with normal weight, insulin secretion was only slightly altered. In contrast, extracellular vesicles from obese adipose tissue specifically transferred proteins and nucleic acids to the pancreas, where they greatly increased the release of insulin. As a result, blood glucose levels dropped.
Stemmer explains the significance of the results: "There is a gap in our understanding of the development of type 2 diabetes. When we are overweight or obese, for example, our body cells in muscle or adipose tissue react less sensitively to insulin; we talk about insulin resistance. In this very early stage of type 2 diabetes, our pancreas has to secrete more insulin, for example after a meal, to keep blood glucose levels in the normal range. But how do the pancreatic beta cells recognize that there is insulin resistance such that they need to provide more insulin?"
The researcher continues, "An increase in insulin secretion is very beneficial in this early stage of type 2 diabetes and leads to the body being able to maintain its blood glucose level at a normal level. Many overweight and obese people manage to do this for decades, and the disease never develops. Extracellular vesicles from the fat cells appear to play an important role in this process."
"Overall, extracellular vesicles have great potential for application in the diagnosis and treatment of a wide variety of diseases," says Stemmer. "Our ongoing studies aim to specifically load the vesicles to be able to use them for therapeutic purposes."
In other studies, researchers in Augsburg and Munich are currently developing new methods for being able to use extracellular vesicles circulating in the blood for a minimally invasive investigation of organ functions. "The close networking of our Institute for Theoretical Medicine with the Augsburg University Hospital and the Helmholtz Munich Research Center creates optimal conditions for such innovative research approaches, which will ultimately serve the well-being of diabetes patients," says Stemmer.
More information: Konxhe Kulaj et al, Adipocyte-derived extracellular vesicles increase insulin secretion through transport of insulinotropic protein cargo, Nature Communications (2023). DOI: 10.1038/s41467-023-36148-1
Journal information: Nature Communications
Provided by Universität Augsburg