Credit: Journal of Medicinal Chemistry (2023). DOI: 10.1021/acs.jmedchem.2c01771
Building a new precision cancer treatment comes with no shortage of scientific challenges. Now, a group of University of Florida researchers have made a significant discovery in the effort to develop potent, highly targeted chemotherapies for breast and other cancers.
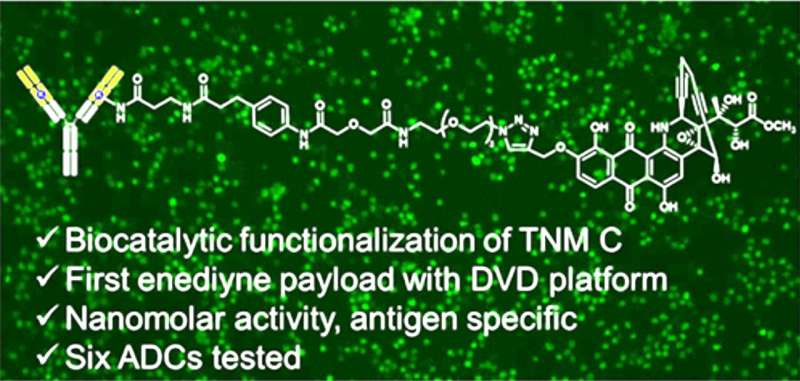
Scientists at The Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology have spent years developing antibody-drug conjugates, or ADCs, that combine a protective protein and a cancer-killing drug. Findings published recently in the Journal of Medicinal Chemistry detail the first time a particular type of cancer-killing "payload" has been used for ADC development.
The laboratory findings in leukemia cells are a key step in creating new medicinal payloads that can be altered and translated into ADCs to treat different types of cancer, the researchers said.
"We have shown we can precisely generate ADCs in a manner that is different than what anyone has done in the clinic. It's the ability to customize every single portion of ADCs in a pretty rapid fashion," said Andrew D. Steele, Ph.D., a postdoctoral research associate and co-first author of the paper.
Steele likens an ADC to a biological guided missile: The antibody is a targeting system that takes aim at the cancer. A potent, toxic compound is similar to a warhead, killing cancer cells by obliterating its DNA. A chemical linker acts like vital missile hardware, connecting the antibody and its chemical payload.
Building a new cancer-killing ADC posed several significant scientific hurdles, Steele said. Attaching payloads to antibodies in a controlled manner is quite challenging. Antibodies often have to be re-engineered for different types of cancers. Also, only six payloads are being used in federally approved ADCs. The current findings address all of those challenges, Steele said. The research was co-led by Ben Shen, Ph.D., a professor and director of the Natural Products Discovery Center, and professor Christoph Rader, Ph.D., both of The Wertheim UF Scripps Institute.
The team's discovery also solves a long-standing challenge of having to create a new ADC for different cancers.
"We now are able to address breast cancer or attack leukemia cells. Otherwise, we would need to synthesize an ADC again and again for each cancer type. This gets us around that problem," said Alexander F. Kiefer, Ph.D., a postdoctoral fellow and co-first author of the paper.
After demonstrating effectiveness against leukemia cells in the study, the team hopes to move into mouse-model testing. One important future target is breast cancer that is driven by a protein known as human epidermal growth factor receptor 2, or HER2, Kiefer said. About 20% of breast tumors have elevated levels of HER2, according to the American Cancer Society.
More information: Andrew D. Steele et al, Application of a Biocatalytic Strategy for the Preparation of Tiancimycin-Based Antibody–Drug Conjugates Revealing Key Insights into Structure–Activity Relationships, Journal of Medicinal Chemistry (2023). DOI: 10.1021/acs.jmedchem.2c01771
Journal information: Journal of Medicinal Chemistry
Provided by University of Florida