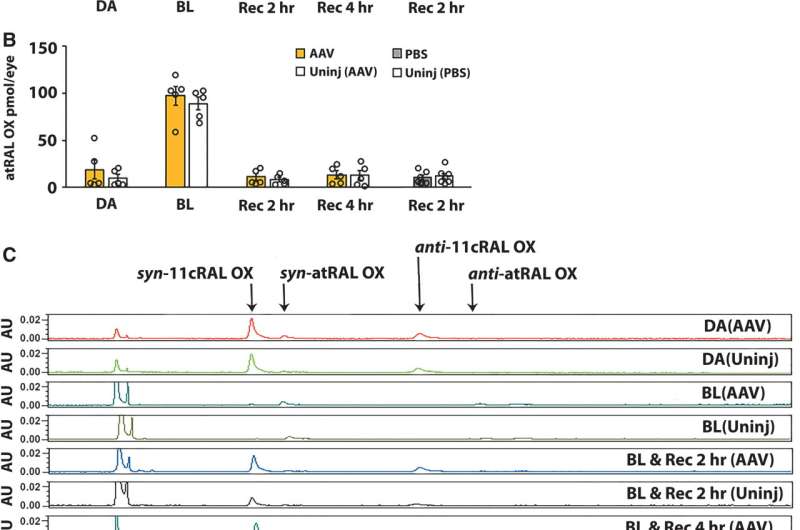
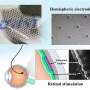
HPLC analysis of dark-adapted and light-adapted heterozygous D477G KI mice showed similar levels of chromophore in eyes that received AAV-RPE65 or PBS or were uninjected, whereas recovery of 11-cis retinal at 2 h post bleaching was significantly increased only in eyes that received AAV-RPE65. (A) 11-cis retinal and (B) all-trans retinal, quantified as the sum of syn- and anti-retinal oximes, in eyes from mice at 6–8 months postinjection and uninjected controls that were dark-adapted, bleached, and recovered in the dark for 2 or 4 h. AAV-RPE65, filled yellow bars; uninjected, open bars (n = 5). PBS injected, filled gray bars; uninjected, open bars (n = 7). (C) Representative HPLC chromatograms. *p = 0.013, **p = 0.003. 11cRAL OX, 11-cis-retinal oxime; atRAL OX, all-trans-retinal oxime; BL, bleached; DA, dark-adapted; HPLC, high-performance liquid chromatography; PBS, phosphate-buffered saline; Rec, recovered. Credit: Human Gene Therapy (2023). DOI: 10.1089/hum.2022.240
A new study shows that total RPE65 protein levels in mice with autosomal dominant retinitis pigmentosa were doubled following subretinal delivery of adeno-associated virus (AAV)-RPE65 gene supplementation. The study is published in Human Gene Therapy.
Debra Thompson, from the University of Michigan Medical School, and coauthors, assessed gene supplementation in mice with a monoallelic mutation encoding a rare D477G RPE65 variant (D477G KI mice). Total RPE65 protein levels are decreased in heterozygous D477G KI mice. After treatment, recombinant RPE65 localized specifically to the retinal pigment epithelium and was stable for at least 6 months post-injection.
In addition, report the investigators, "rates of recovery of the chromophore 11-cis retinal after bleaching were significantly increased in eyes that received AAV-RPE65, consistent with increased RPE65 isomerase activity." They add, "It remains of significant interest to determine whether increase RPE65 expression can reduce the disease burden associated with D477G RPE65."
"While patients with inherited retinal dystrophy due to RPE65 deficiency can benefit from the FDA approved gene therapy, it has been very unclear whether patients with autosomal dominant retinitis pigmentosa due to the D477G RPE65 mutation could be treated in a similar way," says Editor-in-Chief Terence R. Flotte, MD, Celia and Isaac Haidak Professor of Medical Education and Dean, Provost, and Executive Deputy Chancellor, University of Massachusetts Chan Medical School.
"This study provides important initial proof-of-principle data in support of gene supplementation as a treatment for patients with this mutation."
More information: Kecia L. Feathers et al, Gene Supplementation in Mice Heterozygous for the D477G RPE65 Variant Implicated in Autosomal Dominant Retinitis Pigmentosa, Human Gene Therapy (2023). DOI: 10.1089/hum.2022.240
Journal information: Human Gene Therapy
Provided by Mary Ann Liebert, Inc