This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Second generation gene therapy for alpha 1-antitrypsin deficiency
Researchers report on the safety of a gene therapy to treat the common autosomal recessive hereditary disorder alpha 1-antitrypsin (AAT) deficiency in a new article in Human Gene Therapy.
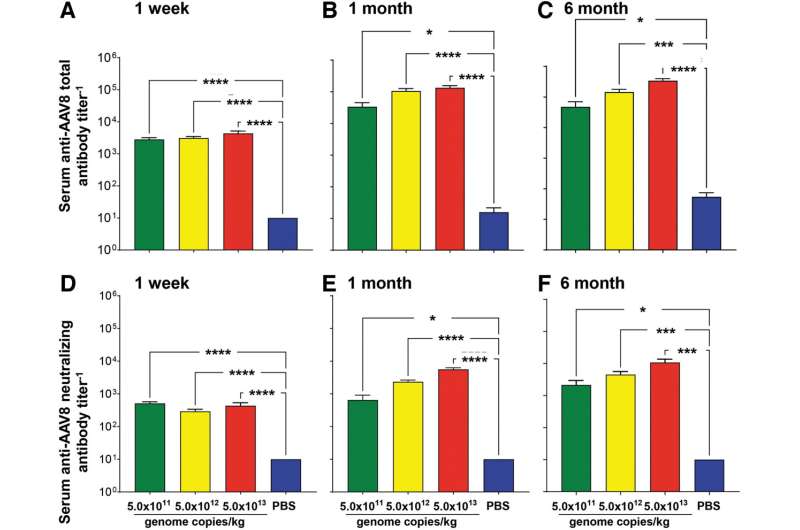
In ATT deficiency, neutrophil proteases destroy the lung parenchyma, the portion of the lungs involved in gas exchange. The result is a high risk for the early onset of emphysema. Ronald Crystal, MD, from Weill Cornell Medicine, and co-authors, have developed an adeno-associated virus (AAV) serotype 8-based gene therapy for AAT deficiency that codes for an engineered variant of AAT. In the current study, they evaluate the safety of intravenous administration of this gene therapy, called AAV8hAAT(AVL), in mice at three doses compared to control mice.
"The data demonstrates that intravenous administration of AAV8hAAT(AVL) is safe with no significant adverse effects attributed to AAV8hAAT(AVL) vector at any dose," conclude the investigators. These findings are "consistent with the requirements for proceeding to a clinical study."
"Improving the potency of gene therapy vectors is a crucial step in developing therapies that will be effective at doses that are safe and feasible to manufacture," says Editor in Chief Terence R. Flotte, MD, Celia and Isaac Haidak Professor of Medical Education and Dean, Provost, and Executive Deputy Chancellor, University of Massachusetts Medical School. "Accomplishing this for AAT deficiency is particularly important given the high levels of circulating anti-protease activity that are required to help these patients."
More information: Jonathan B. Rosenberg et al, Safety of Intravenous Administration of an AAV8 Vector Coding for an Oxidation-Resistant Human α1-Antitrypsin for the Treatment of α1-Antitrypsin Deficiency, Human Gene Therapy (2023). DOI: 10.1089/hum.2022.192