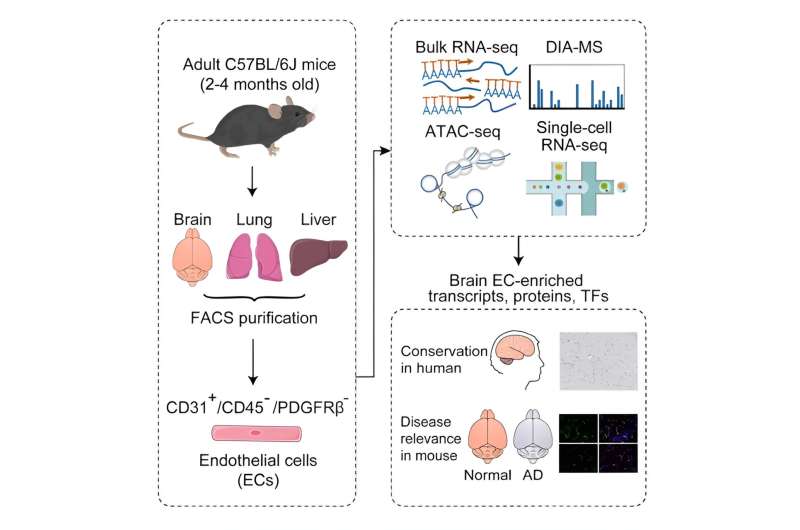
Graphical abstract of this study. Credit: Prof. Chang, from Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113392
A team of researchers from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences (CAS), Sun Yat-Sen University, and Stanford University, has revealed a comprehensive molecular atlas of the adult mouse blood–brain barrier (BBB) at the endothelial cell (EC) level, shedding new light on BBB function in health and neurodegenerative disease. The study was published in Cell Reports.
Blood vessels within the central nervous system (CNS) exhibit unique anatomical and functional characteristics known as the BBB. This protective feature shields vulnerable neurons from potentially harmful substances in the bloodstream, playing a critical role in CNS homeostasis.
"The BBB is primarily characterized by various physiological properties of CNS ECs, but the molecular basis for these properties remains incompletely understood," said Prof. Calvin J. Kuo from Stanford University, the corresponding author of this study.
In this study, researchers employed integrated multi-omics analyses to comprehensively profile the transcriptome, proteome, and chromatin accessibility of adult brain ECs. They validated the identified brain EC-enriched proteins and transcription factors in normal mouse and human brain tissue and assessed their expression changes in mice with Alzheimer's disease.
Initially, flow cytometry isolated adult mouse brain and peripheral tissue ECs with high purity. Subsequent RNA-seq and data-independent acquisition-mass spectrometry (DIA-MS) analyses identified selectively enriched transcripts and proteins in brain ECs, revealing a partial dissociation between transcript and protein levels. This emphasizes the importance of scrutinizing protein levels when investigating a gene's role in BBB formation and regulation.
Additionally, researchers identified TCF/LEF, SOX, and ETS families as the top transcription factors regulating the BBB, providing a theoretical foundation for further epigenetic research on BBB mechanisms. Through comprehensive screening, eight proteins and three transcription factors with high expression in adult mouse brain capillary ECs were identified, showcasing molecular similarities between mouse and human BBB.
Finally, to uncover disease-related molecular signatures of BBB, researchers examined the expression levels of brain EC-enriched proteins and transcription factors in a mouse model of Alzheimer's disease. Significant downregulations were observed, revealing potential molecular alterations in the BBB in Alzheimer's disease, warranting further investigations.
"Our work will propel efforts to understand and modulate the adult BBB, providing potential molecular targets for treating neurological disorders and enhancing drug delivery to the CNS," said Prof. Chang Junlei from SIAT, another corresponding author of this study.
More information: Min Yu et al, Integrative multi-omic profiling of adult mouse brain endothelial cells and potential implications in Alzheimer's disease, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.113392
Journal information: Cell Reports
Provided by Chinese Academy of Sciences