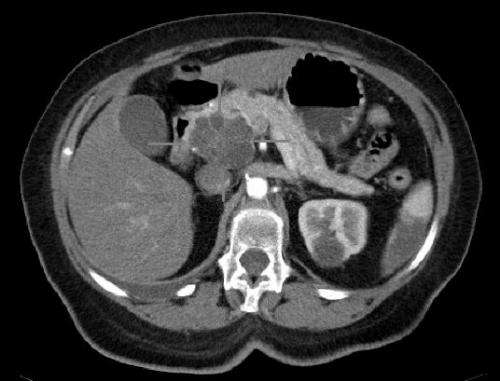
Axial CT image with i.v. contrast. Macrocystic adenocarcinoma of the pancreatic head. Credit: public domain
Clinicians at Georgetown University's Lombardi Comprehensive Cancer Center reported promising preliminary findings based on outcomes in the first six patients with metastatic pancreatic cancer enrolled in a Phase II clinical trial of the experimental drug BXCL701 in combination with the immunotherapy drug pembrolizumab (Keytruda). Immunotherapy drugs alone have not shown to be responsive to pancreatic cancer.
The findings are being presented at the American Society of Clinical Oncology 2024 annual meeting in Chicago on June 1, 2024 (LBA4132).
BXCL701, made by BioXcel Therapeutics, is an oral drug called an immune activator. It acts by inflaming the microenvironment surrounding the tumor and thereby augmenting the activity of immunotherapies like pembrolizumab. BXCL701 has received Orphan Drug Designation from the U.S. Food and Drug Administration in pancreatic cancer and three other cancer types: acute myelogenous leukemia, stage IIb to IV melanoma, and soft tissue sarcoma.
Pancreatic cancer is the third leading cause of cancer death. The five-year survival rate for all stages of the disease is 13% but there's no early detection method and therefore patients are often diagnosed with later stage disease. For those with disease that has spread beyond the pancreas, the five-year survival rates are an extremely low 3%.
"Pancreatic cancer is a devastating disease that is difficult to treat with standard methods, including chemotherapy," says Benjamin Adam Weinberg, MD, an associate professor of medicine at Georgetown Lombardi and lead investigator of this trial.
"Using immunotherapy alone to harness the body's own immune system has also generally not worked due to the inability of immune cells to infiltrate pancreatic tumors due to dense fibrous tissue that walls tumors off microscopically from the immune system. Hence the need to find another approach."
In pre-clinical mouse studies, published in 2021, researchers led by Louis Weiner, MD, director of Georgetown University's Lombardi Comprehensive Cancer Center, showed that when combined with an immunotherapy drug, BXCL701 boosted the animals' natural immune systems, slowing or even stopping pancreatic tumor growth.
The mouse studies found evidence that tumors had been flooded by natural killer immune cells, a sign that the drug had accomplished its goal of making cancer cells receptive to the immune system. In short order, these mouse studies led directly to this first-in-human trial of the drug in pancreatic cancer.
For the initial part of the trial looking primarily at the safety aspect of the drug combination, three women and three men with a median age of 57 were enrolled. One patient showed no signs of disease progressing after 18 weeks on the trial and one patient had stable disease at nine weeks but was not yet evaluable for the 18-week landmark. The progression-free survival rate (no change or even signs of regression in the tumor) as determined by imaging was 50%.
Three patients had significant reductions in a marker that indicates the presence of pancreatic cancer, CA19-9, with 100%, 97% and 73% reductions. Weinberg says that CA19-9 is the best marker they have in pancreatic cancer, but it is not as good a screening test as PSA is for prostate cancer. He believes, however, that it can be useful for monitoring disease activity in patients with advanced cancers. Not every patient's tumor makes CA19-9 but so far in this study all patients have had elevated CA19-9 levels.
According to Weinberg, the main side effect so far is low blood pressure, which can be mitigated by giving BXCL701 at a lower dose during the first week of treatment. The clinicians are also seeing anemia, nausea, and immune-related arthritis, which has responded to oral steroids.
"Historically, no one has responded to immunotherapy in pancreatic cancer so even one response and another with stable disease could be early signs of efficacy," says Weinberg. "A third patient had an initial drop in their CA19-9 marker even though they had disease progression on imaging, and this may be more early evidence that this drug combination has anti-cancer effects."
More information: Phase II trial of BXCL701 and pembrolizumab in patients with metastatic pancreatic ductal adenocarcinoma (EXPEL-PANC): Preliminary findings., American Society of Clinical Oncology 2024
Provided by Georgetown University Medical Center