This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Pancreatic cancer vaccine plus immunotherapy and antibody spark immune system response in pancreatic cancers
![J1568 study treatment schema.Eligible patients with clinically resectable PDA received the first priming study treatment Cy-GVAX-based therapy (alone [Arm A], + PD-1 [Arm B], + PD-1 and CD137 [Arm C]) 2 weeks before the surgical resection, and the 2nd priming treatment 6–10 weeks following definitive surgical resection. Patients began SOC adjuvant therapy ~4 weeks following the 2nd study treatment. SOC adjuvant chemotherapy was administered as per the standard of care at the time at the discretion of the primary treatment oncologist. The 3rd (and up to 6th) priming study treatment was administered every 28 days beginning four weeks after the completion of SOC adjuvant therapy. Study treatment was given as follows: Day 1–Cyclophosphamide (Cy) 200 mg/m2 IV (Arms A, B, C), nivolumab (PD-1) initially, 3 mg/kg, and later 480 mg IV following approval of every 4 week flat dose (Arms B, C), urelumab (CD137) 8 mg IV (Arm C Only); Day 2–GVAX intradermal (Arms A, B, C) was injected equally into six intradermal areas in both lower limbs and the non-dominant upper limb. This study began randomized enrollment to Arms A and B in March 2016. In October 2018, the study protocol was amended to add Arm C (due to limited supply of urelumab, Arm C had to enroll consecutively) as well as an optional “extended-treatment” phase. In this “extended-treatment” phase, all patients with no evidence of recurrence following the initial six priming doses of study treatment were given the option to receive additional Cy-GVAX every 12 weeks (up to 2 additional treatments), and, for Arm B and Arm C participants only, nivolumab (without ureulmab) every 4 weeks (up to six additional treatments). Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-39196-9 Pancreatic cancer vaccine plus immunotherapy and antibody spark immune system response in pancreatic cancers](https://scx1.b-cdn.net/csz/news/800a/2023/pancreatic-cancer-vacc.jpg)
Giving patients with operable pancreatic cancers a three-pronged combination immunotherapy treatment consisting of the pancreatic cancer vaccine GVAX, the immune checkpoint therapy nivolumab and urelemab, an anti-CD137 agonist antibody treatment, is safe, increases the amount of cancer-killing immune system T cells in the tumors and appears effective when given two weeks prior to cancer-removal surgery, according to new research directed by Johns Hopkins investigators.
A description of the work was published online June 20 in the journal Nature Communications.
This study, led by researchers at the Johns Hopkins Kimmel Cancer Center, the Bloomberg~Kimmel Institute for Cancer Immunotherapy and the Johns Hopkins University School of Medicine, is the latest from an ongoing platform trial formed in 2015 to study immunotherapy treatments before surgery (neoadjuvant) and after surgery (adjuvant) for patients with pancreatic cancer. This format enables researchers to use data generated by the trial to advance development of immunotherapies for pancreatic cancer within the same study.
In this most recent part of the trial, 10 participants received the combination treatment. The median disease-free survival—the amount of time after treatment during which no cancer is found—was 33.51 months, and the median overall survival—time to death—was 35.5 months. These were higher than found in previous arms of the trial that tested the pancreatic cancer vaccine alone and in combination with nivolumab, but because of the small number of patients, the results did not have statistical significance.
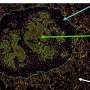
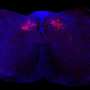
The tumor specimens studied in the recent arm also had much higher amounts of cancer-killing immune cells than specimens from patients given only the vaccine or the vaccine plus nivolumab.
The results suggest that this therapy combination warrants further study in a larger clinical trial, says senior study author Lei Zheng, M.D., Ph.D., co-director of the Pancreatic Cancer Precision Medicine Center of Excellence and professor of oncology at the Johns Hopkins University School of Medicine.
The platform trial has two purposes regarding pancreatic cancer treatments given during the two-week "window of opportunity" prior to surgery, Zheng says. First, it allows the immunotherapies to teach the patient's immune cells how to respond to tumors, so they can continue surveillance later if the cancer recurs. Second, it enables investigators to see, by evaluating the tumors removed during surgery, how well the tumors respond to the treatment.
A fourth arm of the trial, studying anti-interleukin-8 neutrophil-blocking antibodies in pancreatic tumors, is ongoing.
More information: Thatcher Heumann et al, A platform trial of neoadjuvant and adjuvant antitumor vaccination alone or in combination with PD-1 antagonist and CD137 agonist antibodies in patients with resectable pancreatic adenocarcinoma, Nature Communications (2023). DOI: 10.1038/s41467-023-39196-9