This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers develop new compound designed to treat drug-resistant acute myeloid leukemia
Researchers at Purdue University's College of Science have developed a patent-pending compound called HSN748 to treat drug-resistant acute myeloid leukemia (AML). AML is a cancer that begins in bone marrow and sometimes metastasizes to the central nervous system, liver, lymph nodes, spleen and testicles.
Herman Sintim leads the team that has developed the compound. He is a Distinguished Professor in Chemistry and the Richard B. Wetherill Professor of Chemistry and Drug Discovery in the James Tarpo Jr. and Margaret Tarpo Department of Chemistry. He also is on the faculty of the Purdue Institute for Cancer Research and the Purdue Institute for Drug Discovery.
Sintim's development team collaborated with a group including Reuben Kapur and Baskar Ramdas at the Indiana University School of Medicine and KinaRx Inc. to validate the effectiveness of the compound. Kapur is the director of the Herman B Wells Center for Pediatric Research and co-leader of the Hematopoiesis and Hematologic Malignancies program at the IU Melvin and Bren Simon Comprehensive Cancer Center. Ramdas is an assistant research professor of pediatrics.
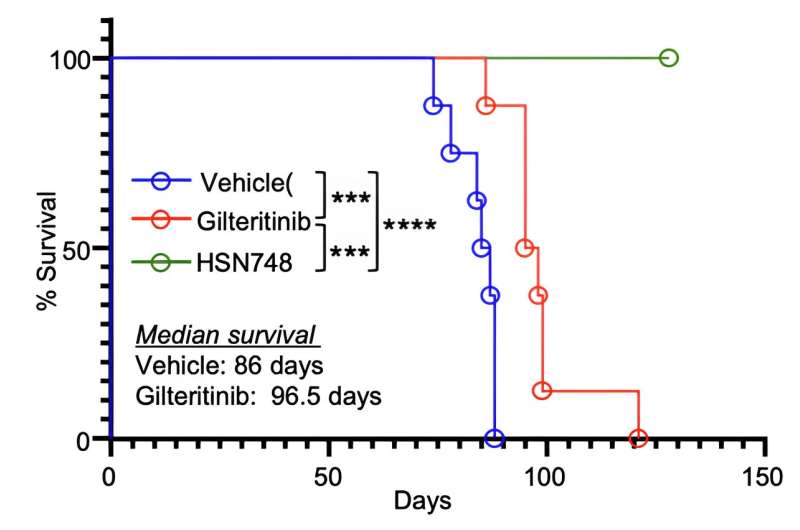
They demonstrated that HSN748 effectively treated mice implanted with patient-derived, drug-resistant AML with 100% survivability after 120 days. Their co-authored paper of research results has been published in the Journal of Clinical Investigation.
Sintim disclosed HSN748 to the Purdue Innovates Office of Technology Commercialization, which has applied for a patent to protect the intellectual property.
AML metrics and traditional treatments
The National Cancer Institute estimates 20,800 new cases of AML will be diagnosed in 2024, which would represent 1% of new cancer cases in the U.S. It is most often diagnosed in people between the ages of 65 and 74, with a median age of 69. Although uncommon, AML can occur in children. The five-year relative survival rate is 31.9%.
Sintim said, "One of the best U.S. Food and Drug Administration-approved drugs right now to treat AML is called gilteritinib (Xospata). But patients have developed genetic mutations in an enzyme called FLT3 that could render gilteritinib ineffective."
Ramdas said, "Despite the widespread occurrence and clinical importance of FLT3 mutations in causing AML, treatment options tailored to this genetic anomaly are scarce. Our goal was to identify new and powerful inhibitors targeting the mutations, particularly those resistant to currently approved FDA options."
How HSN748 works
Sintim and his team have identified inhibitors, or blocking agents, that target FLT3 gene mutations, the most common mutation in AML. Sintim used the three-dimensional atomic structure of FLT3 to address the challenges of drug resistance.
"The three-dimensional atomic structure guided the molecular design and synthesis of HSN748 so it fits perfectly into the active site of drug-resistant mutants of FLT3, like a hand fitting into a glove," Sintim said. "The enzymatic activity of FLT3 is crucial for AML cancer cell survival, so filling in the active site with HSN748 kills the enzyme's activity and also kills the cancer cells."
Sintim continued, "Remarkably, while all mice that had been implanted with gilteritinib-resistant human patient AML samples and were treated with HSN748 were alive by day 120, none of the animals treated with gilteritinib survived past day 120, demonstrating the superiority of this investigational drug over the FDA-approved drug."
Kapur said, "Our preclinical study results have shown incredible promise, and we're excited to keep the momentum going so AML patients can have more resilient options."
Sintim is one of the founders of KinaRx, which has licensed HSN748 from the Purdue Innovates Office of Technology Commercialization. He said the next stage to develop the compound is clinical trials.
"We are at the planning stage and looking to secure investments from different types of investors," he said.
More information: Baskar Ramdas et al, Alkynyl nicotinamides show antileukemic activity in drug-resistant acute myeloid leukemia, Journal of Clinical Investigation (2024). DOI: 10.1172/JCI169245