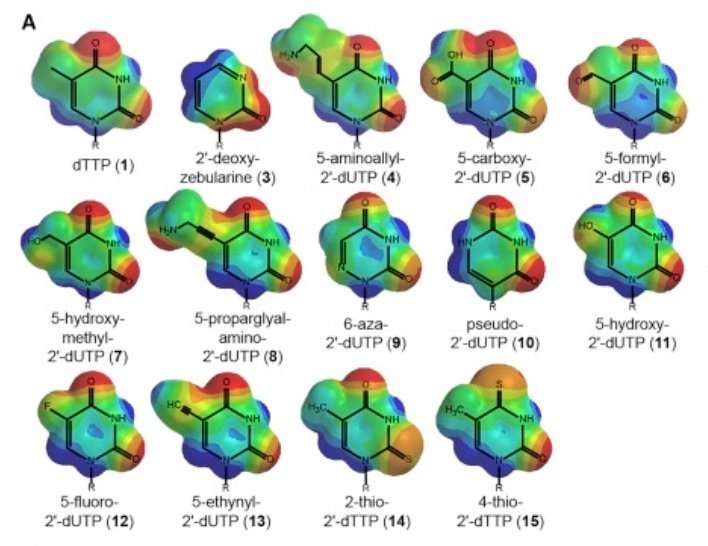
Pyrimidine analogs. Credit: Xuehuo Zeng et. al.
Telomerase is a reverse transcriptase that uses an RNA template to synthesize telomeres. These repeat sequences bind special proteins that fold the ends of chromosomes back onto themselves to create a stable cap. When this cap is damaged, the cell often interprets the exposed DNA as a double strand break and attempts to 'repair' it.
The problem with repairing chromosome ends that aren't really broken is that chromosomes can be anomalously fused together. This creates further instabilities that can lead to cancer. Fortunately, cells activate additional DNA damage responses to check further progression through the cell cycle and if need be, gracefully dismantle the cell altogether.
Telomeres progressively shrink with each cell division. They also contain a lot of guanine that tends to get oxidized. After embryonic development, most cells turn off telomerase. There are several important exceptions to this. For example, stem cells that must continually generate new sperm, or even some long-lived memory B cells keep telomerase active. A more insidious exception is tumor cells—some 90 percent of them somehow figure out how to re-activate telomerase to perpetuate their growth.
A logical response to treat aberrantly activated telomerase in cancer would be to block it again. Unfortunately, this strategy hasn't panned out because during the time it takes for telomerase-inhibited cancer cells to run out of telomeres, they miraculously come up with alternative ways to grow telomeres, which is called alternative lengthening of telomeres. Apparently, in its altruistic truce of multicellularity, nature has been down this same route many times before.
A paper just published in the journal Cell Reports suggests another way forward, namely, to use the selective expression of telomerase in cancer cells as a Trojan horse by delivering non-native nucleotides that would build dysfunctional telomeres. The beauty of this approach is that, if done right, these artificial telomeres activate the lethal DNA damage response to kill the tumor.
The researchers demonstrated that telomerase incorporates the fluorinated pyrimidine analog 5-FdUTP into chromosome ends and kills cancer cells in just a few days. The way it works is that 5-FdUTP gets added in place of thymidine by telomerase. This substitution sufficiently perturbs the construction of the proper protein amenities that telomeres need to create the T-loop that caps chromosomes. These proteins include POTS1 and the shelterin complex.
The rationale for trying 5-FdUTP in the first place comes from a new appreciation for how its sister molecule 5-fluorouracil (5FU) works in cells. 5FU is a toxic but highly successful chemotherapy used systemically or as a cream for skin cancers. Its cytotoxicity has traditionally been attributed to depleting nucleotide pools by blocking thymidylate synthase. This step requires that 5FU is first converted to its monophosphate form. As we have recently seen, proper maintenance of nucleotide levels is not only critical in controlling the immune system, but also GABAergic neurotransmission.
It is now appreciated that the full mechanism of 5FU includes the incorporation of its analogs into both RNA and DNA, hence the idea that maybe the reverse transcriptase telomerase may utilize it. Given that this approach seems to work, how useful might it be in actual human therapies?
Obviously, any normal cells that also use telomerase would be also be expected to be hurt by 5-FdUTP. Perhaps this would be an acceptable liability. Another consideration is that with the proliferation of genomic knowledge, there are now clear genetic indications for 5FU treatment which might pertain equally to 5-FdUTP. Not everyone metabolises these analogs identically, nor are they distributed in the same way by transporters. Increasingly, the larger nucleotide ecosystem must be understood less as a gas tank macroscopically available to the body, but rather more as microscopic fuel injectors that control every compartment.
This conception is now embodied within neural systems in particular in the form of the nucleosidome, a complex metabolic and molecular cross-talk between the extracellular nucleotide cascade system and the intracellular nucleoside salvage. At the fore is the role of mitochondria as sensors and conduits of nucleotide status across synapses. It is not just GABAergic transmission that has a hand in this, it is all neurotransmitter systems. For example, nucleotide flow through mitochondria specific to the serotonergic system via ANT1 transporters has recently been shown to be the cause of such high-level diseases as bipolar disorder.
The bridge to making these kinds of discoveries invariably comes from low-level anecdotes pertaining to more tangible disorder. In this particular case, it was observations about mitochondrial disorders like chronic progressive external ophthalmoplegia (CPEO) caused by a specific variant in the mitonuclear genome. Here, it was big data that came to the rescue to elucidate the more casual links to an esoteric mental disorder.
Nucletides and nucleosides have already proven their mettle in treating various mitochondrial DNA depletion disorders. Their many analogs are also now invaluable as chemotherapies for cancer, and also for big diseases like AIDS. As these systems are increasingly targeted in more nuanced metabolic diseases whose most visible and debilitating symptoms are more 'in the head,' so to speak (consider the nail-biting disorder of purine salvage in Lesh-Nyhan syndrome), it will be critical to deploy these powerful therapies bearing some of the more global possible effects in mind.
More information: Xuehuo Zeng et al. Administration of a Nucleoside Analog Promotes Cancer Cell Death in a Telomerase-Dependent Manner, Cell Reports (2018). DOI: 10.1016/j.celrep.2018.05.020
Journal information: Cell Reports
© 2018 Medical Xpress