Dr. Jeff Biernaskie, PhD Credit: UCalgary Faculty of Veterinary Medicine
People who suffer severe burns or extensive skin injuries are often left to live with extreme scarring, disfigurement, and skin that feels chronically tight and itchy. That's because the body's healing processes have evolved to focus on preventing infection by quickly closing up wounds, rather than regenerating or restoring normal skin tissue.
New research led by Dr. Jeff Biernaskie, Ph.D., has made an exciting leap forward in understanding how skin heals, which could lead to drug treatments to vastly improve wound healing. The study, published in the scientific journal Cell Stem Cell, was co-led by Dr. Sepideh Abbasi, Ph.D., Sarthak Sinha, MD/Ph.D. candidate and Dr. Elodie Labit, Ph.D., postdoctoral fellow.
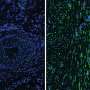
"We identified a specific population of progenitor cells that reside within the dermis, the deep connective tissue of the skin. Progenitor cells, are unique in that they are able to undergo cell division and generate many new cells to either maintain or repair tissues. Following injury, these dermal progenitors become activated, proliferate and then migrate into the wound where they generate nearly all of the new tissue that will fill the wound, both scar and regenerated tissue," says Biernaskie, professor of stem cell biology in the University of Calgary Faculty of Veterinary Medicine (UCVM), and the Calgary Firefighters Burn Treatment Society Chair in Skin Regeneration and Wound Healing.
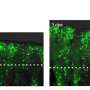
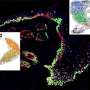
Biernaskie's intensive study, five years in the making, offers new knowledge on why certain dermal cells are able to regenerate new skin, rather than disfiguring scar tissue. Using cutting-edge genomics techniques to profile thousands of individual cells at different times after injury, the research team compared scar-forming versus regenerative zones within skin wounds.
"Remarkably, we found that although these cells come from the same cellular origin, different microenvironments within the wound activate entirely different sets of genes. Meaning, the signals found within 'regenerative zones' of the wound promote re-activation of genes that are typically engaged during skin development. Whereas, in scar-forming zones these pro-regenerative programs are absent or suppressed and scar-forming programs dominate."
Working with these findings, the researchers then showed it's possible to modify the genetic programs that govern skin regeneration.
"What we've shown is that you can alter the wound environment with drugs, or modify the genetics of these progenitor cells directly, and both are sufficient to change their behavior during wound healing. And that can have really quite impressive effects on healing that includes regeneration of new hair follicles, glands and fat within the wounded skin," says Biernaskie.
This research offers critical insights into the molecular signals that drive scar formation during wound healing and it identifies a number of genetic signals that are able to overcome fibrosis and promote true regeneration of adult skin.
"This proof of principle is really important, because it suggests that the adult wound-responsive cells do in fact harbor a latent regenerative capacity, it just simply needs to be unmasked," says Biernaskie. "Now, we are actively looking for additional pathways that may be involved. Our hope is to develop a cocktail of drugs that we could safely administer in humans and animals to entirely prevent genetic programs that initiate scar formation in order to greatly improve the quality of skin healing."
More information: Sepideh Abbasi et al, Distinct Regulatory Programs Control the Latent Regenerative Potential of Dermal Fibroblasts during Wound Healing, Cell Stem Cell (2020). DOI: 10.1016/j.stem.2020.07.008
Journal information: Cell Stem Cell
Provided by University of Calgary