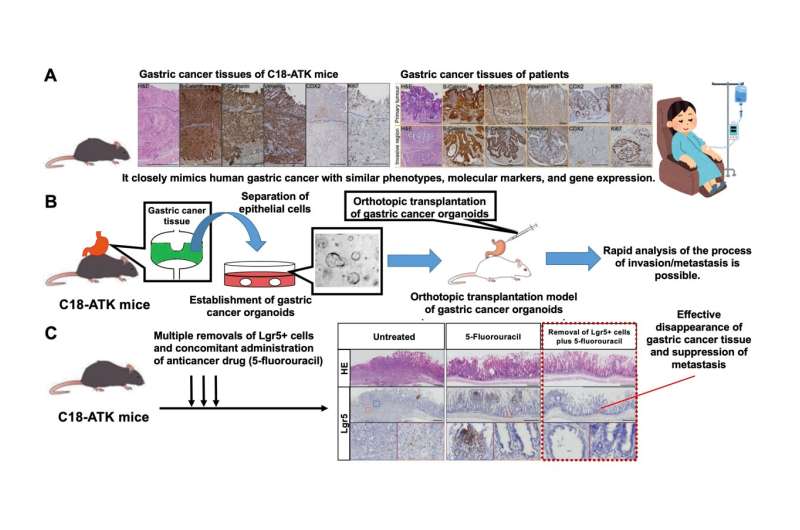
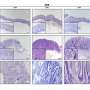
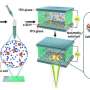
A. A Cldn18-IRES-CreERT2 driver that selectively introduces mutations in mouse gastric epithelium. Researchers developed a new mouse model (C18-ATK) that reproduces human metastatic gastric cancer by combining Wnt with Kras or Trp53 mutations reported in human gastric cancer. This mouse model accurately reproduces human advanced gastric cancer and can be an excellent model for gastric cancer research. B. Orthotopic transplantation models using gastric cancer organoids established from gastric cancer tissue of C18-ATK mice. By using these transplantation models, the time needed for analysis can be significantly shortened, and high-quality, large-scale analysis becomes possible. C. The combination of removal of Lgr5+ gastric cancer stem cells and an anticancer drug, 5-fluorouracil, significantly suppressed the progression of gastric cancer. It was suggested that a breakthrough treatment for advanced gastric cancer could be developed by targeting Lgr5+ gastric cancer cells that are also present in human gastric cancer. Credit: Kanazawa University
Researchers have succeeded in establishing a mouse model that develops gastric cancer closely resembling advanced human gastric cancer. Using this model, they have discovered gastric cancer stem cells, i.e. Lgr5+ gastric cancer cells, essential for the development, maintenance, and metastasis of cancer. The study provides an experimental system that enables detailed analysis of highly malignant gastric cancer and is expected to lead to the development of a breakthrough treatment for advanced human gastric cancer.
In Japan, about one million people are newly diagnosed with cancer each year. Gastric cancer is the third leading cause of death from cancer, with more than 40,000 Japanese dying of it annually. Chemotherapy using anticancer drugs is used for advanced gastric cancer, but there is still no completely effective treatment. One reason is that there are no mouse models that can reproduce the development and malignant transformation of human gastric cancer in vivo. So, even now, the mechanisms of gastric cancer development and malignant transformation are not fully understood and effective treatments for malignant gastric cancer have not been established.
Recently, the existence of cancer stem cells has been suggested as one of the causes of drug resistance exhibited by malignant cancer. Cancer stem cells have multipotency and self-renewal ability. Even if the surrounding cancer cells disappear following chemotherapy, the stem cells survive, regenerate cancer cells, and cause the cancer to recur. Until now, however, the existence of cancer stem cells has not been unequivocally established and therefore detailed analysis of gastric cancer stem cells has not been performed.
Therefore, for the development of effective treatments for malignant gastric cancer, it is essential to elucidate the malignancy mechanisms causing invasion and metastasis in vivo by establishing a mouse model that closely mimics human gastric cancer, taking into account the characteristics of gastric cancer stem cells present in gastric cancer tissue including their interaction with the surrounding microenvironment.
The present study was performed by an international research team consisting of scientists of the Division of Epithelial Stem Cell Biology, Cancer Research Institute, Kanazawa University, and scientists from A*Star, Singapore. The research team newly established Cldn18-IRES-CreERT2 mice that could induce mutations selectively in the gastric epithelium. By crossing these with mice that could induce mutations in a WNT signaling gene and a RAS signaling gene or Trp53 gene (as reported in the TCGA gastric cancer database), the team succeeded in developing a new gastric cancer mouse model that could reproduce human metastatic gastric cancer. By comparing and analyzing in detail the gastric cancer tissues generated in these mice and the gastric cancer tissues from patients, it was confirmed that the characteristics and gene expressions observed in human advanced gastric cancer were accurately reproduced. This indicates that their newly established gastric cancer mouse model can be an invaluable resource for basic and preclinical research on gastric cancer. In addition, by orthotopically transplanting organoids established from mouse gastric cancer tissue into the mouse stomach, the team has developed an orthotopic transplantation model that reproduces the metastatic process of gastric cancer and enables large-scale and rapid analysis with high reproducibility.
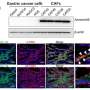
Through detailed analysis using these models, the team has found that gastric cancer cells expressing the Lgr5 gene, known as a target gene for Wnt signaling, play an important role in the development and progression of gastric cancer. Also, by investigating the contribution of Lgr5+ gastric cancer cells to gastric cancer metastasis using a drug that removes Lgr5+ gastric cancer cells in vivo, they have revealed that Lgr5+ gastric cancer cells are in fact gastric cancer stem cells essential for gastric cancer metastasis. Furthermore, they have clarified that the progression and metastasis of gastric cancer can be effectively suppressed in vivo by using an anticancer drug, 5-fluorouracil, in combination with the removal of Lgr5+ gastric cancer stem cells.
This study has revealed that Lgr5+ gastric cancer stem cells play important roles in the development, progression, and metastasis of gastric cancer. In addition, it has been clarified that the synergistic effect of an anticancer drug and gastric cancer stem cell removal can significantly suppress the progression and metastasis of gastric cancer. The results suggest great clinical significance for the development of a therapeutic method targeting Lgr5+ gastric cancer cells that are also present in human gastric cancer tissue. Furthermore, the new gastric cancer mouse models the team has established may be applicable to elucidation of the mechanism of gastric cancer development, progression, and metastasis, screening of new diagnostic and therapeutic agents, and preclinical research. Further studies could serve to develop an effective treatment for metastatic gastric cancer by targeting gastric cancer stem cells.
More information: A. Fatehullah et al, A tumour-resident Lgr5+ stem-cell-like pool drives the establishment and progression of advanced gastric cancers, Nature Cell Biology (2021). DOI: 10.1038/s41556-021-00793-9
Journal information: Nature Cell Biology
Provided by Kanazawa University