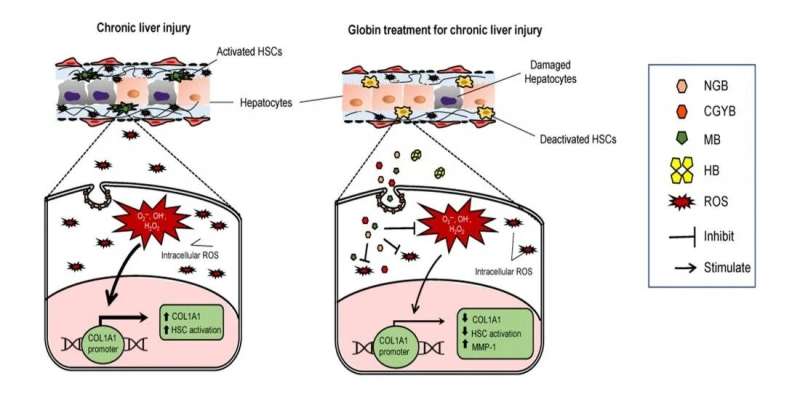
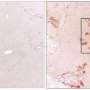
MB, NGB, and CYGB, but not HB, were endocytosed into the HSCs via endocytosis pathway, scavenged intracellular ROS, and suppressed COL1A1 promoter activity, resulting in HSC deactivation and inhibition of collagen production in vitro and in vivo. Globin therapy could be utilized as anti-fibrotic therapy in human liver cirrhosis. Credit: Osaka City University
Researchers have discovered in mice an additional use of globins as an intravenous drug that can delay liver fibrosis progression. Last year, the application of recombinant human cytoglobin (CYGB) as a protein therapeutic agent against liver damage and cirrhosis was published in Hepatology by Professor Norifumi Kawada's research group. Here, they studied the antifibrotic properties of the globin family members hemoglobin (HB), myoglobin (MB), and neuroglobin (NGB) in comparison with CYGB. All globins demonstrated greater antioxidant capacity than glutathione in cell-free systems. Interestingly, all globins, except HB, could enter the cells and inhibit the collagen synthesis leading to a suppression of fibrosis development both in vitro and in vivo. The study was published in the journal Redox Biology.
Anti-fibrotic therapy remains an unmet medical need in human chronic liver diseases. A research team led by Professor Norifumi Kawada, Osaka City University (OCU), reported the anti-fibrotic function of globin family members in hepatic stellate cells (HSCs), the main cell type involved in liver fibrosis. In mice with advanced liver fibrosis, myoglobin (MB), (neuroglobin) NGB and (cytoglobin) CYGB injection can suppress liver inflammation and fibrosis.
The functions of globin family members have been extensively studied, focusing primarily on the specific tissues in which these proteins are expressed: hemoglobin (HB) in erythrocytes, MB in muscle cells, NGB in nervous tissues, and CYGB in pericytes and fibroblasts. Beyond the well-established oxygen-binding respiratory functions of heme-containing proteins, all globins are also known to be involved in the regulation of harmful reactive oxygen species (ROS), protecting cells from oxidative stress.
"Liver fibrosis occurs after repetitive and long-lasting injury or inflammation in the liver. These injuries are accompanied with the accumulation of ROS that activate HSCs, followed by collagen production," explains Dr. Kawada.
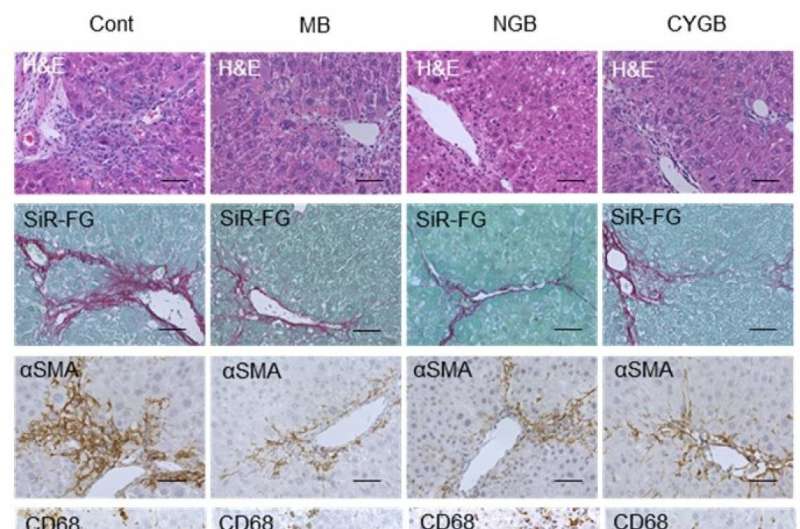
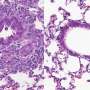
Intravenously injected MB, NGB, and CYGB markedly suppressed liver inflammation, fibrosis and oxidative cell damage in fibrotic mice without adverse effects. Cont. untreated control; MB, NGB, CYGB: intravenous administration of MB, NGB, CYGB. H&E, hematoxylin and eosin staining; SiR-FG, Sirius red-fast green staining; SMA, stellate cell activation marker; CD68: macrophage marker; Neu-Dapi, Neutrofil marker – DAPI nuclear staining; 4-HNE, oxidative cell damage marker, 4-hydroxynonenal. Credit: Osaka City University
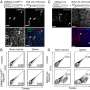
Along with Dr. Le Thi Thanh Thuy, Ph.D. fellow Vu Ngoc Hieu, and colleagues, Dr. Kawada observed that when they culture human HSCs under globin treatments, MB, NGB, and CYGB enter the cellular organelles, hunt the intracellular harmful ROSs, and reduce the direct signal regulating the production of collagen. "Results showed the antioxidant capacity of the globins to be greater than the well-documented glutathione and even vitamin C," continues Dr. Kawada.
This is great news for three members of the globin family, but what happened to HB? "We noticed that hemoglobin did not enter the cell," explains Dr. Thuy, "we speculate this is due to size as HB is four times the size of its monomer siblings."
In the next set of experiments, Dr. Kawada and his group generated a mouse model of advanced liver fibrosis using chemical agents and applied MB, NGB, and CYGB by intravenous injection. Interestingly, the therapeutic protein dramatically suppressed liver inflammation and fibrosis without any side effects. Ph.D. fellow Hieu points out, "in addition to the liver, we focused on possible side effects with the neighboring kidney. Creatinine levels remained normal throughout the treatment."
With this discovery, published in the journal Redox Biology, the research team hopes to establish a foothold to a potential therapy for liver fibrosis in the near future.
More information: Vu Ngoc Hieu et al, Capacity of extracellular globins to reduce liver fibrosis via scavenging reactive oxygen species and promoting MMP-1 secretion, Redox Biology (2022). DOI: 10.1016/j.redox.2022.102286
Journal information: Hepatology
Provided by Osaka City University