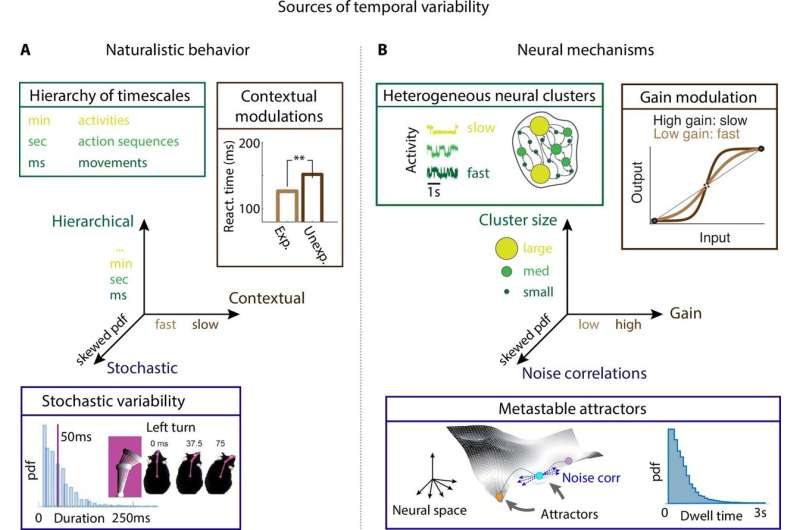
Neural mechanisms underlying the temporal organization of naturalistic animal behavior. (A) Three sources of temporal variability in naturalistic behavior: hierarchical (from fast movements, to behavioral action sequences, to slow activities and long-term goals), contextual (reaction times are faster when stimuli are expected), and stochastic (the distribution of ‘turn right’ action in freely moving rats is right-skewed). (B) Neural mechanisms underlying each source of temporal variability: hierarchical variability may arise from recurrent networks with a heterogeneous distribution of neural cluster sizes; contextual modulations from neuronal gain modulation; stochastic variability from metastable attractor dynamics where transitions between attractors are driven by low-dimensional noise, leading to right-skewed distributions of attractor dwell times. Credit: eLife (2022). DOI: 10.7554/eLife.76577
For years, neuroscience experiments have depended on carefully controlled conditions. Mice run in place on tiny treadmills, rather than freely scurrying. Or they're meticulously trained to do easy-to-measure tasks that don't mimic their behaviors in the wild. Even in human experiments, people sit still inside an fMRI machine and look at images on a screen.
These kinds of experiments have given scientists a solid foundational understanding of how the brain works. But they also erase much of the complexity of carrying out even seemingly simple behaviors. Scientists still don't understand how our visual system lets us perform everyday actions like finding a pencil on a cluttered desk or running down a rocky trail.
So as technology has improved, neuroscientists are now pushing the boundaries of traditional experiments and studying the brain in more naturalistic ways. UO neuroscientist Cris Niell is part of this growing movement. In two recent papers, his team has developed ways to study mouse vision that more realistically represent the way animals navigate the world beyond the lab.
"Rather than thinking of vision like an eye exam or Zoom meetings on a computer screen, we are trying to study how vision works in the real, three-dimensional world, where we move around and interact with people and objects," Niell said.
In the first paper, published in eLife, Niell's team evaluated how mice judged distance. They challenge the animals to jump between two platforms across a large gap. The platforms varied in their distance apart, so for each jump, mice had to scope out the situation and decide how dramatic of a leap to prepare for.
The animals didn't rely solely on binocular visual cues, the researchers found. Mice could still make the leap with one eye covered, which removes the slight difference in images between two eyes that often helps people sense depth. Instead, Niell's team thinks the mice may have been taking advantage of a phenomenon called motion parallax: the effect in which farther-away objects appear to move more slowly than close-by ones.
They noticed that mice who had one eye covered spent more time scouting the gap by moving their heads up and down, much like a cat scoping out whether to jump to the top of a bookshelf. The head movements let the mice use motion parallax to judge distance in the absence of binocular depth cues, the researchers propose. A more controlled experimental setup in which an animal's head is held in place wouldn't have captured that nuance.
"There's been a lot of skepticism about mice's visual capabilities, and I think a lot of the research so far has been focused on binocular vision because it's something we relate to a lot," said Phil Parker, a former postdoctoral researcher in Niell's lab who led the experiments and is now starting his own lab at Rutgers University. "But if you compare their performance over the two conditions, the fact that they don't lose performance shows those (monocular) cues are powerful."
In a second paper, published in Neuron, the team designed a system to record mice's brain activity while exploring a large arena. They fitted mice with a head-mounted camera, like a tiny GoPro. The camera recorded both the mouse's eye and its field of view, while electrodes simultaneously captured brain activity.
Then, the researchers developed a machine learning tool to sync recordings from the electrodes with the mouse's movement and eye positioning. They could determine what the mouse was looking at and how its brain was responding at any moment in time.
That experiment let them tap into the complexity of translating neural activity into a visual scene. For the mouse, "neural activity isn't just dependent on what you see; it's also dependent on where you're looking," said graduate student Elliott Abe, who led the development of the machine learning analysis.
That is, the brain factors in both eye position and head position, so the neural response to seeing the same object directly in front of you is different than the response if you have to look left or right to see it. Combining behavioral research with neural recording lets researchers better understand how those different kinds of signals get combined in the brain.
Niell's team plans to build on the techniques and findings from the two studies in their future work in mice, with likely insights for the human brain, too.
"I think what's really cool about this direction is that by trying to study the mouse for what the mouse is, rather than imposing human-centric behaviors and tasks on the animal, it actually allows us to relate the neuroscience more to humans than we could before," Parker said.
Since the brains of all animals need to solve similar challenges, such as figuring out how far away something is, studying this in the mouse's natural behavior allows researchers to generalize to the types of things that humans do naturally as well.
As Niell and colleagues wrote in a recent review paper in Current Biology, "natural behavior is the language of the brain." And in contrast to typical laboratory experiments, it's what the brain evolved to do. Natural behaviors can therefore provide a type of Rosetta stone for understanding how vision works across the animal kingdom, including in humans.
More information: Luca Mazzucato, Neural mechanisms underlying the temporal organization of naturalistic animal behavior, eLife (2022). DOI: 10.7554/eLife.76577
Shih-Yi Tseng et al, Shared and specialized coding across posterior cortical areas for dynamic navigation decisions, Neuron (2022). DOI: 10.1016/j.neuron.2022.05.012
Cory T. Miller et al, Natural behavior is the language of the brain, Current Biology (2022). DOI: 10.1016/j.cub.2022.03.031
Journal information: Current Biology , Neuron , eLife
Provided by University of Oregon