Associate Professor Stella Valenzuela and PhD student Heba Al Khamici. Credit: Lisa Aloisio
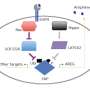
Although known to regulate fundamental cellular processes in humans, including cell growth, division and programmed cell death, the protein group known as chloride intracellular channel (CLIC) proteins is yet to be fully understood.
Now a discovery by a group of researchers led by UTS Associate Professor Stella Valenzuela and PhD student Heba Al Khamici has opened the door on a previously unknown function of the CLIC family of proteins.
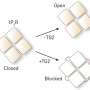
Associate Professor Valenzuela said that up until now members of the CLIC proteins had been assigned one principal function in the body as ion channels – these ion channels regulating the movement of ions across cell membranes.
"To use an analogy, ion channels act like gates that open and close, moving materials like sodium and potassium ions into and out of cells," she said. "This process is an important one that regulates cell growth, cell division or cell death.
"We have now discovered that in addition to this activity these proteins are also capable of carrying out enzymatic activity within cells – essentially the process of speeding up reactions inside cells. This multifunctional activity is referred to as 'moonlighting'."
The CLIC proteins are found in most cells in the human body and given their prominence, this discovery of their moonlighting activity provides scientists with a key to help decipher the map of drug targets for disease and new markers for early detection of cancers and metabolic disorders.
"This new function is like a code breaker that may unravel new functions within cells in curing or treating certain diseases," Associate Professor Valenzuela said.
"People are always looking for ways to discriminate the normal from pathological, so if we've identified elevated levels of a CLIC protein in cancer cells or in blood circulation then that could be a nice biomarker that could be used in medicine and diagnostics."
She said the discovery of a new function for the proteins also meant scientists could now delve further into what their critical role in cells might be.
"Initially, I discovered one of these proteins (CLIC 1) in the early 1990s. It was my PhD project and when we discovered it, we found that it had ion channel activity.
"A number of years ago another research group found CLIC proteins had structural similarities to a family of enzymes, but apart from that, no one had shown that the CLIC proteins had enzymatic activity.
"This development is very exciting because it opens up an entire new realm for us to start exploring the functions of these proteins in a way we had previously ignored, and in fact hadn't realised was there.
"The interesting thing here is that these proteins now are known to have two distinct functions – typically the dogma states 'one protein; one function', but now there is further evidence that proteins can have multiple functions which adds to the diversity in protein function and how they collaborate to carry out the many different cellular functions essential for life."
The research paper Members of the Chloride Intracellular Ion Channel Protein Family Demonstrate Glutaredoxin-Like Enzymatic Activity was published on 12 January 2015 in the journal PLOS ONE.
More information: "Members of the Chloride Intracellular Ion Channel Protein Family Demonstrate Glutaredoxin-Like Enzymatic Activity." PLoS ONE 10(1): e115699. DOI: 10.1371/journal.pone.0115699
Journal information: PLoS ONE
Provided by University of Technology, Sydney