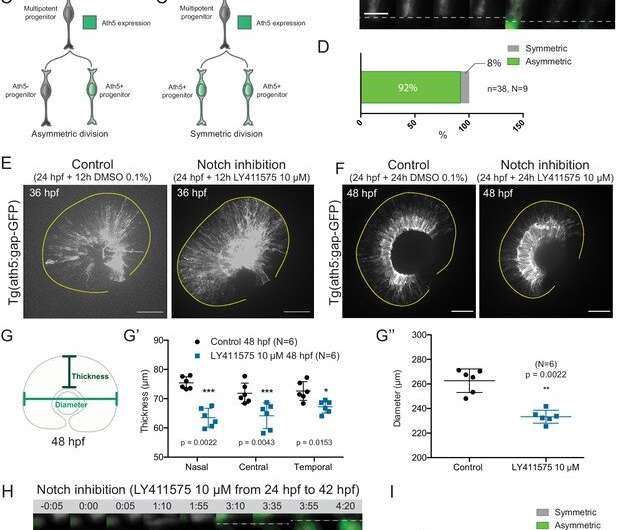
Asymmetric cell divisions generate Ath5+ progenitors in a Notch-dependent manner. (A) Schematic of mosaic Ath5 expression in the retina (green) at 28 hpf. Injection of ath5:GFP-CAAX construct at 1 cell stage. (B) Example of an asymmetric multipotent progenitor division with regards to Ath5 expression onset. hsp70:H2B-RFP (nuclei, grey), hsp70:mkate2-ras (cell membrane, grey), ath5:GFP-CAAX (Ath5, green). Dashed lines show apical and basal sides of the retinal neuroepithelium. Scale bar, 10 μm. Magenta and yellow dots label sister cells. (C–C’) Schematics of multipotent progenitor cells dividing asymmetrically (C) or symmetrically (C’) with regards to Ath5 expression. (D) Distribution of asymmetric vs symmetric divisions observed in live imaging experiments. N = number of embryos, n = number of divisions. (E) Ath5+ cells (grey) at 36 hpf in control (left) vs Notch inhibition (right). Scale bar, 50 μm. The yellow line delimits the apical side of the retinal neuroepithelium. (F) Ath5+ cells (grey) at 48 hpf in control (left) vs Notch inhibition (right). Scale bar, 50 μm. The yellow line delimits the apical side of the retinal neuroepithelium. (G) Schematic of retinal neuroepithelium measurements at 48 hpf, (G’) retinal thickness control vs Notch inhibition, (G’’) retinal diameter control vs Notch inhibition. Mann-Whitney test used for comparison. Vertical bars represent standard deviation. (H) Example of symmetric progenitor division upon Notch inhibition. hsp70:H2B-RFP (nuclei, grey), ath5:GFP-CAAX (Ath5, green). Dashed lines show apical and basal sides of the retinal neuroepithelium. Scale bar, 10 μm. Magenta and yellow dots label sister cells. (I) Distribution of asymmetric vs symmetric divisions observed in live imaging experiments in Notch inhibition compared to controls.
Researchers at the Instituto Gulbenkian de Ciência and the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden, Germany, have discovered that in the developing retina, and important part of the central nervous system, the divisions leading to the first differentiating neurons are asymmetric and that this asymmetry is necessary to generate the correct types of neurons in the right numbers and proportions.
Balancing proliferation and differentiation in a developing organ are a complex act, especially when these two processes occur at the same time in the same space. The retina is an important interface between the body and the external world: It sits at the back of the eyes and receives and encodes all the visual information, so that the brain can continuously process images.
"For attaining this function, the retina requires a precise balance of different types of neurons organized in several interconnected layers, each receiving, pooling or filtering the visual input," explains Elisa Nerli, first author of the study and researcher at Instituto Gulbenkian de Ciência. "The formation of the different neurons in the correct numbers and proportions is ultimately ensured by balancing cellular proliferation and differentiation during development."
Studying the development of the zebrafish retina, the team led by Caren Norden, principal investigator at the Instituto Gulbenkian de Ciência, discovered that this balancing depends on asymmetric divisions of progenitor cells on their way to making functional neurons. Nerli says, "We further found that the molecular regulation of this process relies on the Notch signaling pathway, since its inhibition interferes with the division asymmetry. We were able to observe that Notch is asymmetrically distributed during cell division. The cell that inherits Notch signaling will continue to proliferate, whereas the other cell will enter a neurogenic lineage."
Overall, this study adds new perspectives to the fundamental understanding of how cellular decisions of proliferation or differentiation can regulate the development of the nervous system. Understanding how the balance between these processes is determined and maintained is important for a better understanding of brain development, in health and in disease.
More information: Elisa Nerli et al, Asymmetric neurogenic commitment of retinal progenitors involves Notch through the endocytic pathway, eLife (2020). DOI: 10.7554/eLife.60462
Journal information: eLife
Provided by Instituto Gulbenkian de Ciencia