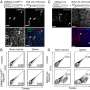
Graphical abstract. Credit: Cancers (2024). DOI: 10.3390/cancers16051060
Neuroblastoma, one of the most common childhood cancers, is classified as a developmental cancer because it arises prenatally during the formation of organs and tissues. It originates from cancer cells that develop in neuroblasts, a type of immature nerve tissue, and primarily affects the adrenal glands.
One of the research focal points of Dr. Josep Samitier's group (Nanobioengineering Group of IBEC), led by Dr. Aránzazu Villasante, is the creation of in vitro neuroblastoma models that replicate its characteristic vasculature in order to search for new biomarkers and develop effective therapies against this type of cancer.
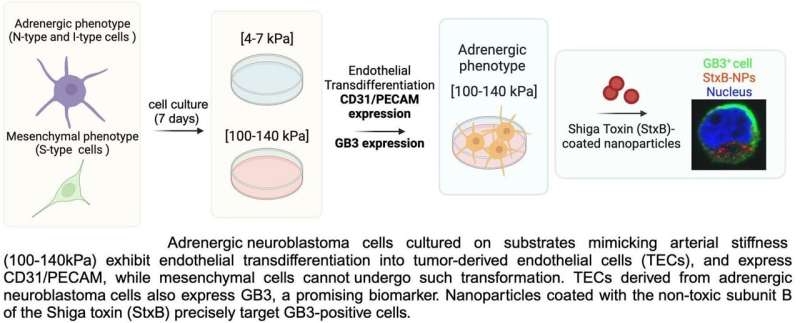
This is important because a hallmark of neuroblastoma is its increased vascularization, which can be mediated by a process known as transdifferentiation, in which cancer cells transform into endothelial cells that form the tumor's blood vessels. This process has been linked to treatment resistance and cancer recurrence.
Dr. Villasante, a senior researcher in the group, is the lead author of two recent studies on this topic, published in the journals In vitro models and Cancers, respectively. These studies describe on how the research team was able to replicate the transdifferentiation process of the neuroblastoma vasculature in in vitro models.
They achieved this in two easily reproducible systems: a simpler 2D model used to explore potential therapeutic targets, and a more complex microfluidic chip designed for drug screening. Additionally, they have identified the biomarker GB3 as a potential therapeutic target for future nanotherapies against neuroblastoma.
"When neuroblastoma cells are cultured on a 2D plate, they do not undergo transdifferentiation as they would under physiological conditions. However, we found that by modifying the plate substrate to simulate the stiffness of human arteries and veins, the cells began to form the characteristic alternative vasculature of neuroblastoma that we were looking for," explains Dr. Villasante, an IBEC researcher affiliated to the University of Barcelona and CIBER-BBN.
Using this model, the researchers were able to confirm that the tumor cells expressed the GB3 biomarker, a cell receptor involved in blood vessel formation, metastasis and drug resistance.
As Dr. Villasante points out, the next step was to carry out a pilot nanoparticle study. "We coated the surface of the nanoparticles with a toxin known to bind to GB3 and observed that they directly targeted the neuroblastoma cells." These results highlight the potential of GB3 as a therapeutic target for the development of future targeted nanotherapies.
The next step will be to transfer these studies to the more complex microfluidic model, which will allow drug screening and selection of those with the highest potential before moving to in vivo studies.
The study was carried out in collaboration with the Pediatric Cancer Center Barcelona at SJD Barcelona Children's Hospital and the Institute of Health Research (IDIVAL) at the University of Cantabria.
More information: Aranzazu Villasante et al, Identification of GB3 as a Novel Biomarker of Tumor-Derived Vasculature in Neuroblastoma Using a Stiffness-Based Model, Cancers (2024). DOI: 10.3390/cancers16051060
Aranzazu Villasante et al, Microfluidic model of the alternative vasculature in neuroblastoma, In vitro models (2024). DOI: 10.1007/s44164-023-00064-x
Journal information: Cancers
Provided by Institute for Bioengineering of Catalonia