Drug tests will prevent repeat of Northwick Park trial
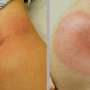
Scientists investigating the 2006 Northwick Park drug-trial disaster that left six healthy volunteers hospitalised say they have developed new pre-clinical tests that could have stopped the trial from ever going ahead.
But Dr Stephen Poole, speaking at the British Pharmacological Society's Winter Meeting in Brighton today (Tuesday), said that research is still "ongoing" to understand why the drug had such an adverse effect in the clinic but not in pre-clinical testing.
Describing the incident as "the most obvious setback for medicines testing since thalidomide", Dr Poole and his colleagues, from the National Institute for Biological Standards and Control (NIBSC) have, with new tests, successfully reproduced the devastating reaction suffered by the volunteers using human cells in the test tube (in vitro).
Standard preclinical in vitro tests on TGN1412 – the immunotherapy drug responsible for the Northwick Park disaster – failed to predict the catastrophic reaction that would occur when TGN1412 was administered to human subjects.
TGN1412 is from a class of drugs developed to re-balance the immune system for the treatment of autoimmune diseases, where the immune system has started to attack the body, such as rheumatoid arthritis and multiple sclerosis.
The Northwick Park disaster resulted in the UK Medicine and Healthcare products Regulatory Agency (MHRA) suspending all clinical trials of immunotherapy drugs and commissioning NIBSC, a government-funded institute, to investigate why both in vitro human cell tests and in vivo animal tests failed to predict the human immune system's response to the drug.
"With hindsight testing this drug in man was a mistake, but at the time the standard required pre-clinical tests failed to predict the effects it would have on the six volunteers," said Dr Poole, who said that the NIBSC's group's second paper on TGN1412 was due to be published shortly.
"While we are still investigating why the effect of this drug was so catastrophically different in the clinic than in pre-clinical testing, we have at least managed to develop new pre-clinical tests that should help us to avoid such outcomes in the future."
Speaking at the British Pharmacological Society (BPS) conference, Dr Poole identifies new pre-clinical in vitro testing of immunotherapy drugs that should help prevent any repetition of the disastrous events that were witnessed at Northwick Park.
These measures include ensuring that such drugs are not tested solely on immune (white blood) cells in isolation. The NIBSC group has shown that having a mixed human cell culture of white blood cells and endothelial cells – the cells that line blood vessels – is a much better indicator of how this type of drug will react in vivo.
The NIBSC group has also developed a technique that dries the drug onto a plastic surface, rather than testing it on cells as a solution in water, which has proven to be a far more reliable indicator of how the drug will react in the human body.
"The aim of our research is to improve the preclinical testing of immunotherapy drugs on human cells in vitro, as well as to establish why the antibody was not toxic in pre-clinical testing," said Dr Poole.
"We have made significant progress in designing new in vitro tests that hopefully will avoid the consequences that occurred at Northwick Park; indeed such tests could prevent harmful drugs of this type even reaching the animal-testing stage."
Immunotherapy drugs have the potential to be incredibly important in the treatment of diseases that have so far eluded medical advances, including many forms of cancer, so it is vital that the scientific community has its faith in clinical trials and in immunotherapy fully restored.
Source: University of Manchester