A change of heart: Researchers reprogram brain cells to become heart cells
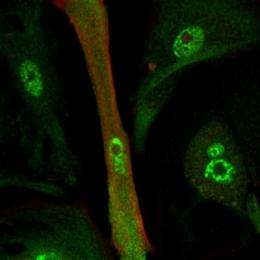
For the past decade, researchers have tried to reprogram the identity of all kinds of cell types. Heart cells are one of the most sought-after cells in regenerative medicine because researchers anticipate that they may help to repair injured hearts by replacing lost tissue. Now, researchers at the Perelman School of Medicine at the University of Pennsylvania are the first to demonstrate the direct conversion of a non-heart cell type into a heart cell by RNA transfer. Working on the idea that the signature of a cell is defined by molecules called messenger RNAs (mRNAs), which contain the chemical blueprint for how to make a protein, the investigators changed two different cell types, an astrocyte (a star-shaped brain cell) and a fibroblast (a skin cell), into a heart cell, using mRNAs.
James Eberwine, PhD, the Elmer Holmes Bobst Professor of Pharmacology, Tae Kyung Kim, PhD, post-doctoral fellow, and colleagues report their findings online this week in the Proceedings of the National Academy of Sciences. This approach offers the possibility for cell-based therapy for cardiovascular diseases.
"What's new about this approach for heart-cell generation is that we directly converted one cell type to another using RNA, without an intermediate step," explains Eberwine. The scientists put an excess of heart cell mRNAs into either astrocytes or fibroblasts using lipid-mediated transfection, and the host cell does the rest. These RNA populations (through translation or by modulation of the expression of other RNAs) direct DNA in the host nucleus to change the cell's RNA populations to that of the destination cell type (heart cell, or tCardiomyocyte), which in turn changes the phenotype of the host cell into the destination cell.
The method the group used, called Transcriptome Induced Phenotype Remodeling, or TIPeR, is distinct from the induced pluripotent stem cell (iPS) approach used by many labs in that host cells do not have to be dedifferentiated to a pluripotent state and then redifferentiated with growth factors to the destination cell type. TIPeR is more similar to prior nuclear transfer work in which the nucleus of one cell is transferred into another cell where upon the transferred nucleus then directs the cell to change its phenotype based upon the RNAs that are made. The tCardiomyocyte work follows directly from earlier work from the Eberwine lab, where neurons were converted into tAstrocytes using the TIPeR process.
The team first extracted mRNA from a heart cell, then put it into host cells. Because there are now so many more heart-cell mRNAs versus astrocyte or fibroblast mRNAs, they take over the indigenous RNA population. The heart-cell mRNAs are translated into heart-cell proteins in the cell cytoplasm. These heart-cell proteins then influence gene expression in the host nucleus so that heart-cell genes are turned on and heart-cell-enriched proteins are made.
To track the change from an astrocyte to heart cell, the team looked at the new cells' RNA profile using single cell microarray analysis; cell shape; and immunological and electrical properties. While TIPeR-generated tCardiomyocytes are of significant use in fundamental science it is easy to envision their potential use to screen for heart cell therapeutics, say the study authors. What's more, creation of tCardiomyoctes from patients would permit personalized screening for efficacy of drug treatments; screening of new drugs; and potentially as a cellular therapeutic.
















