Structure of Parkinson's disease protein identified
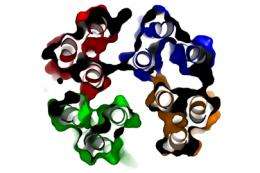
A team of researchers from the Petsko-Ringe and Pochapsky laboratories at Brandeis have produced and determined the structure of alpha-synuclein, a key protein associated with Parkinson’s disease.
Their findings, recently published in Proceedings of the National Academy of Sciences (PNAS), provide information that may someday be used to produce a new kind of treatment for the incurable degenerative brain disorder.
While people with Parkinson’s diseases exhibit many obvious symptoms such as tremors and weakness of face and throat muscles, definitive diagnosis of Parkinson’s comes post mortem, when alpha-synuclein proteins become denatured and form clumps called Lewy bodies in the brain.
“We don’t really know whether this is a side effect or whether it’s the cause of Parkinson’s disease, but we do know that the clumps of proteins are always there,” says Thomas C. Pochapsky, professor of chemistry and one of the authors of the paper. Pochapsky’s lab was responsible for examining the protein using nuclear magnetic resonance, a sort of MRI for molecules, housed at the Landsman Research Facility.
Alpha-synuclein is found in large quantities in the brain. Its association with Parkinson’s disease has stirred curiosity since it was discovered in 1997.
“Nobody knows what it does, but there’s a lot of it,” says Pochapsky. “The question is whether the unfolded or coagulated Lewy body protein just represents the pathological form of something that’s normally doing something.”
To explore that question, the scientists wanted to find out what the form alpha-synuclein is in before it turns into Lewy body clumps, figuring that if it is possible to stabilize, the progression of Parkinson’s disease could be either slowed or reversed.
“Even if we don’t know what it is, we at least want to know in what form alpha-synuclein protein should be under normal conditions,” says Pochapsky.
Gregory Petsko, professor of biochemistry, compares alpha-synuclein to an origami bird that is benign when intact but dangerous if it unfolds. This knowledge could someday lead to the development of therapies that act like glue, helping the protein keep its shape. While some drugs perform in this manner in the treatment of other diseases, the possibility of one for Parkinson’s has not been investigated because, until now, scientists thought the Parkinson’s protein had no structure. The possibility that the protein actually has a structure and that this form of the protein is benign, means that such an approach can now be considered.
The research has the potential to create a paradigm shift, a dramatic change in how science views a given phenomenon.
More than half a million Americans suffer from Parkinson's disease and, according to the National Institutes of Health, about 50,000 new cases are reported annually. The altering of alpha-synuclein is also believed to be involved in Lewy Body Dementia, a relative of Parkinson’s that affects another one million Americans.
Quyen Q. Hoang, who was a post-doctoral researcher in the Petsko-Ringe lab, and is continuing with the research in his own lab at Indiana University School of Medicine, played a key role in developing the methodology for producing this protein.
Jeffrey Agar’s lab then used mass spectrometric studies, a method of determining molecule composition. Last, using nuclear magnetic resonance, Pochapsky’s lab examined the structure of the tetramer solution.
“We were able to establish that alpha-synuclein is actually a tetramer, meaning that four of these protein molecules stick together while moving around,” says Pochapsky. “We’ve found what we think is the normal form of alpha-synuclein, which no one has seen before it has clumped together in the form of Lewy bodies.”
Petsko points out that the ability to make this form of the protein may also shed light on its normal function.
“Synuclein makes up a sizeable percentage of the protein in neurons, but no one really understands what its role in the cell is,” Petsko says. “We hope that this form of the protein may be the form that has the benign function, so biochemists and cell biologists may now be able to figure out what it’s doing.”
Pochapsky explains that while researchers in other institutions have examined alpha-synuclein before, it has not been done with this ultra careful method, which maintains the structure.