Finding a tumor suppressor's groove
The tumor suppressor protein called adenomatous polyposis coli (APC) is a central genetic risk factor for colorectal cancer. In fact, mutations that potentially alter the structure or function of this protein are found in an estimated 85% of human colorectal tumors, which are currently the third highest-ranked cause of cancer-related death worldwide.
Unfortunately, APC is a very large protein with numerous interacting partners, making it a challenge to determine the direct impact of a given mutation. “The mechanisms by which these mutations lead to cancer is still poorly understood,” says Shigeyuki Yokoyama, director of the RIKEN Systems and Structural Biology Center in Yokohama.
Structural analysis offers helpful insights into protein function, but obtaining these data can be difficult with such massive target proteins. Yokoyama and colleagues therefore decided to focus on one particular segment of APC, known as the armadillo repeat (Arm) domain. In particular, they were interested in understanding how Arm interacts with and inhibits Sam68, an APC-binding protein that is believed to contribute to the activation of genes involved with tumorigenesis.
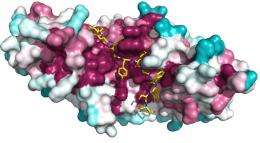
The researchers determined that the Arm domain forms a helical structure, with an L-shaped groove for the recognition of binding partners. The Arm-interacting domain of Sam68, on the other hand, appears to be relatively disorganized until it binds this groove, an association stabilized by a number of interactions between specific amino acids on the two proteins (Fig. 1). Yokoyama and colleagues were subsequently able to confirm the contributions of these residues by systematically introducing mutations and determining their impact on these proteins’ affinity for each other.
With this information in hand, the researchers assessed the potential significance of real-life mutations at these points of interaction. “We were able to map cancer-related APC mutations within this domain and analyze their effects on the structure of APC and on the binding of proteins to APC,” says Yokoyama. Indeed, some of the amino acid changes introduced by these mutations appeared to be directly detrimental to complex formation between APC and Sam68.
Arm interacts with several other important proteins, and Yokoyama’s group plans to perform equally in-depth analyses to characterize the structural bases for these associations. However, he notes that the present findings—in conjunction with data obtained by co-author Tetsu Akiyama at the University of Tokyo—may already offer direct clinical utility. “We think that targeted disruption of the interaction between mutated APCs and Sam68 might be a good strategy to prevent the development of cancer,” says Yokoyama.
More information: Morishita, E.C., et al. Crystal structures of the armadillo repeat domain of adenomatous polyposis coli and its complex with the tyrosine-rich domain of Sam68. Structure 19, 1496–1508 (2011).