Instrumented spinal fusion method impacts infection rate
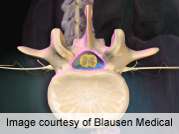
(HealthDay) -- For patients who undergo instrumented spinal fusion, the rates of infection are higher among those who receive posterior lumbar interbody fusion compared with those who receive posterior or posterolateral fusion, according to a study published online May 10 in the Journal of Spinal Disorders & Techniques.
Dong Ki Ahn, M.D. of the Seoul Sacred Heart General Hospital in Korea, and associates conducted a retrospective analysis of 3,084 patients who had instrumental spinal fusion surgeries between 2000 and 2009. The difference in the rates and characteristics of surgical site infections were compared for patients who underwent posterior or posterolateral fusion (Group I; 974 patients) and those who underwent posterior lumbar interbody fusion (Group II; 2,110 patients).
The researchers observed a significant difference in the infection rate between groups I and II (0.3 versus 1.37 percent; P = 0.003). Of the infections in group I, 67 percent were wound infections and 33 percent were osteomyelitis. The infections in group II were mainly osteomyelitis (73 percent), with 23 percent wound infections and 4 percent osteomyelitis combined with wound infection. In the single cage group and mainly local bone grafted group, there was a significantly increased infection rate.
"The infection rate of posterior lumbar interbody fusion was higher than that of posterior or posterolateral fusion," the authors write.
More information:
Abstract
Full Text (subscription or payment may be required)
Copyright © 2012 HealthDay. All rights reserved.