August 1, 2012 report
FDA approves use of electronic chips in medications
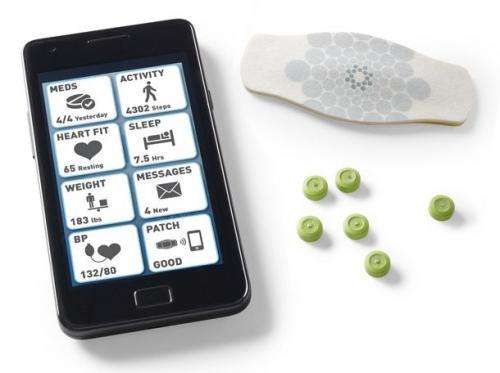
(Medical Xpress) -- The Food and Drug Administration in the United States has approved a request by Proteus Digital Health to allow for the inclusion of tiny digestible microchips into medicines to assist health care workers in monitoring intake of medicines by patients. Previously, the FDA had allowed such microchips only in placebo products. Proteus, the maker of the chips, plans to market them to drug manufactures who can then imbed them in individual pills that allow for electronic reporting to doctors letting them know if and when patients take their medicines.
The microchip, which is described as about the size of a grain of sand, is made of copper, magnesium and silicon, reacts with stomach juices when swallowed along with a pill. Upon reaction, it sends a signal to a patch the patient has applied to their skin where it is relayed to a smartphone. The smartphone then relays the information to the doctor’s office, allowing physicians to track how well a patient adheres to instructions on when and how often they are to take their meds. Proteus insists the aim is not to prod doctors and nurses into becoming nagging nannies, but to provide information that allows doctors to modify the types of medications they prescribe and the schedules to which the patients are asked to follow. Once the microchip has done its job, it dissolves and passes out of the body along with other digested food.
The new technology is being pushed forward by recent reports that have found that just half of all patients take their medicines the way they are supposed to, which of course can reduce their effectiveness. Proteus believes some patients can benefit more than others from the microchips, such as those that take medicines to ward off tuberculosis, diabetics and elderly patients who have difficulty remembering to take their pills or if they’ve already taken them. Making things even more difficult, some have a whole list of pills with different schedules for each. The next logical step would of course be to allow the patient access to the data or to have it routed to a device set near where they keep their medicine, which would both alert them when it’s time to take their meds and to let them know if they can’t remember if they took them or not.
Some suggest this move by the FDA is the first of many likely to come over the next few years, as other technology is waiting in the wings. Coming soon may be swallowed or implanted devices that dose us automatically, sensors that report on other bodily activities, or devices that swim around in our bloodstream monitoring conditions and cleaning out plaque deposits.
More information: proteusdigitalhealth.com/
© 2012 Medical Xpress
















