EU approves Bayer's new long-term contraceptive: firm
German drug maker Bayer said on Wednesday that European Union authorities had given the go-ahead for a new contraceptive which can prevent pregnancies for up to three years.
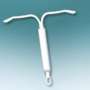
Bayer said in a statement that its healthcare division "has successfully concluded the European registration procedure for its new low dose levonorgestrel-releasing intrauterine system (IUS)."
IUS is "a long-term contraceptive placed in the uterus for the prevention of pregnancy for up to three years."
First launches of the product—which will be marketed under the name "Jaydess"—are expected in the second quarter of 2013 in the EU, the statement said.
Bayer also applied for US marketing authorisation for the product in December 2011.
(c) 2012 AFP