Pilot clinical study into iPS cell therapy for eye disease starts in Japan
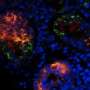
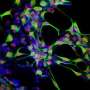
RIKEN is pleased to announce the launch of a pilot study to assess the safety and feasibility of the transplantation of autologous induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) cell sheets in patients with exudative (wet-type) age-related macular degeneration. This study, led by Masayo Takahashi M.D., Ph. D. of the Laboratory for Retinal Regeneration, RIKEN Center for Developmental Biology, and conducted in collaboration with the Institute for Biomedical Research and Innovation with support from the Kobe City Medical Center General Hospital, has been approved to proceed following review by the Ministry of Health, Labour and Welfare and is scheduled to open patient recruitment on August 1, 2013.
Age-related macular degeneration is the most common cause of visual impairment in the elderly, and affects up to 1% of people over 50 years of age in Japan. Wet-type AMD is characterized by progressive damage to the retinal pigment epithelium, a protective layer of non-neural cells located adjacent to the photoreceptors at the back of the eye, due to leakage caused by neovascularization.
Currently available drug treatments for this disease focus on inhibiting neovascularization, but do not repair damage that may have already occurred prior to administration. A number of previous studies have tested the use of RPE cells from various sources, such as fetal tissue or unaffected parts of the RPE, for transplantation but have been complicated by problems of immune rejection or the need for invasive harvesting procedures.
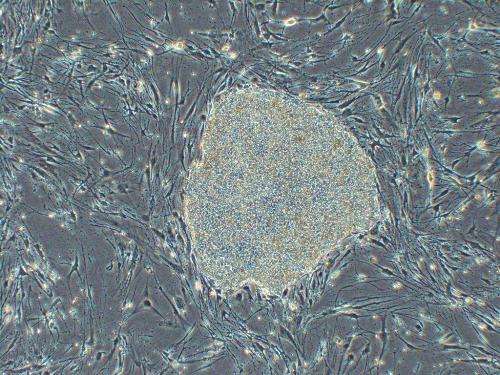
The protocol for Takahashi's new pilot study involves the establishment of autologous iPSCs from each of the research participants, which will then be differentiated into RPE using a novel technology that allows these epithelial cells to be transplanted in monolayer cell sheets without the use of synthetic scaffolds or matrices. The cell sheets will be shaped into 1.3 × 3 mm grafts and transplanted into the affected site of a single eye, following excision of the damaged RPE and neovascular tissues.
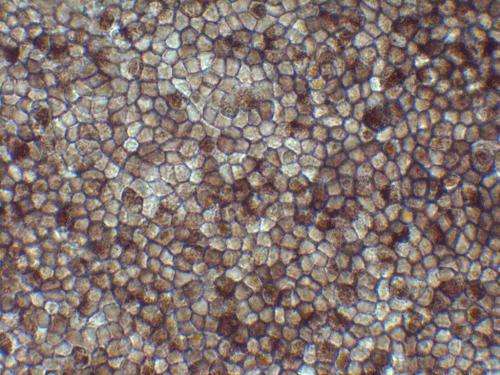
Transplant sites will be monitored closely for functional integration and potential adverse reactions for an initial intensive observation period of one year, and subsequent follow-up observation for three years. Production and validation of the autologous iPSCs and subsequent RPE cell sheets will take approximately 10 months, and will be conducted at a certified clinical-grade cell processing center. The initial three transplants will be conducted at intervals of at least eight weeks; the following three will be performed after a preliminary safety evaluation period.
This pilot study follows on extensive preclinical safety and feasibility testing, including evaluations of cell morphology, physiologic activity, gene expression, immunogenicity, and tumorigenesis in rodent and non-human primate models. The results of these preclinical studies have been submitted for publication in a peer-reviewed journal.
A website describing the research protocol and other details of this pilot study will be launched by RIKEN and the IBRI to promote public awareness and understanding of this research program.