FDA approves GE brain imaging tool for Alzheimer's (Update)
The U.S. Food and Drug Administration on Friday approved a radioactive imaging chemical from General Electric to help screen patients for Alzheimer's disease and dementia.
The drug, Vizamyl, is an injection of radioactive material designed to highlight abnormal brain plaque in medical imaging scans.
Dementia caused by Alzheimer's is associated with buildup of the plaque, known as beta amyloid protein. However, it can also be found in patients who do not have neurological problems.
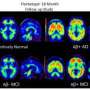
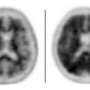
Vizamyl works by binding to the plaque and creating images that show up on positron emission tomography, or PET, scans of the brain.
A negative scan means there is little plaque and the cause of dementia is probably not Alzheimer's, according to an FDA release. A positive scan means the patient has at least some plaque, but does not mean they definitely have Alzheimer's. The injection is intended as one tool to help physicians identify the cause of patient's cognitive decline.
Doctors currently diagnose Alzheimer's disease by observing patients and administering physical and mental tests. The disease is the sixth-leading cause of death in the U.S. and the most common form of dementia, a term for brain disorders that affect memory, judgment and other mental functions.
The Centers for Disease Control and Prevention estimates that Alzheimer's affects 5 million elderly Americans. The agency says that figure may triple in coming years as the people born in the years after World War II age, unless more effective ways are found to prevent the disease.
Alzheimer's attacks neurons in the brain, leading to problems with memory, thinking and behavior. There is no cure for the disease, and scientists are not even sure what causes it.
Vizamyl is manufactured for General Electric Co.'s GE Healthcare division by Medi-Physics Inc. It is the second drug FDA has approved to help screen for Alzheimer's in the last two years. The FDA approved a similar drug called Amyvid from Eli Lilly & Co. in April 2012.
© 2013 The Associated Press. All rights reserved.