Huntington's disease: Hot on the trail of misfolded proteins' toxic modus operandi
Proteins are the workhorses of the cell, and their correctly folded three-dimensional structures are critical to cellular functions. Misfolded structures often fail to properly perform these vital jobs, leading to cellular stress and devastating neurodegenerative disorders such as Alzheimer's, Parkinson's and Huntington's disease.
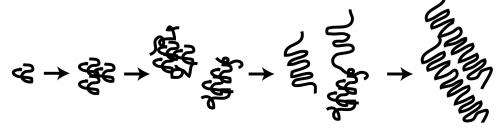
In comparison with the mysteries of Alzheimer's or Parkinson's disease, Huntington's disease has a seemingly simple culprit: an expansion in the polyglutamine (polyQ) tract of a protein called "Huntingtin" (Htt). This polyQ expansion causes the Htt protein to misfold, which triggers a cascade of events—including aggregation of the Htt protein into very stable, fibrillar, amyloid species, and ultimately, neuronal cell death.
"Despite the simplicity of the misfolding involved, we understand very little about why Htt—an essential protein expressed ubiquitously in all human tissue—becomes so toxic when misfolded," said Koning Shen, a grad student working in the Frydman Lab at Stanford University.
Shen will describe her team's multipronged efforts to gain a better understanding of the relationship between protein misfolding, aggregation and cell toxicity at the 58th Annual Biophysical Society Meeting, which takes place Feb. 15-19, 2014, in San Francisco, Calif.
The cause of neuronal toxicity in Huntington's disease remains unknown. Until recently, general consensus had associated fibrillar aggregates with pathogenesis in Huntington's disease. Newer studies, however, point to transient, intermediate species called "oligomers," which occur during the aggregation process, as the key players in neurotoxicity, rather than the fibrillar aggregates.
"Identifying the toxic perpetrators will help explain the pathogenesis of not only Huntington's disease, but perhaps Alzheimer's and Parkinson's as well," explained Shen.
Shen and colleagues also hope to discover which molecular factors may contribute to or ameliorate Htt toxicity. An extended polyQ region is the molecular signature of Htt aggregation, but regions flanking the polyQ tract can also alter the aggregation pathway.
"A molecular chaperone called 'TRiC' can suppress Huntington's disease pathogenesis by binding to one of the polyQ-flanking regions. These flanking regions act as a tool to probe the Htt aggregation pathway to learn how Htt forms toxic aggregate species and how the cell has developed tools to stop it," Shen said. "Altering the regions flanking the polyQ tract could remarkably impact both the aggregation and toxicity of the Huntingtin protein."
Deletions or mutations within these regions may either exacerbate or alleviate aggregation—despite having the same polyQ length. And, Shen pointed out, "fibrillar aggregation and toxicity don't go hand-in-hand amongst these flanking mutants. This finding suggests that there may be toxic intermediate species manifested through the polyQ region, which can be modulated by the polyQ-flanking regions."
Since these flanking region modulations are independent of polyQ length, the ability to use these regions for small molecule or peptide therapeutics delivery will be powerful for Huntington's disease patients, who have already been expressing polyQ-expanded Htt for many years of their lives.
"By manipulating these flanking regions, we may be able to directly influence the aggregation pathway in Huntington's disease patients," said Shen. "Because TRiC binding to a polyQ flanking region suppresses pathogenesis, the interaction between TRiC and Htt shows great potential for therapeutics development."
Recent work has highlighted the ability of a domain of TRiC, called "Apical1," to exhibit TRiC-like effects at suppressing Htt pathogenesis. "This small domain can be more easily adapted into peptide therapeutics and administered to Huntington's disease patients. If developed with an understanding of the toxicity of these misfolded proteins, this next generation of therapeutics may emerge within the next 5 to 10 years," Shen noted.
More information: The presentation "Polyglutamine Flanking Regions in Huntingtin Highlight Key Structural Intermediates Relevant for Molecular Chaperone Interaction and Huntington's Disease Pathogenesis"" by Koning Shen, Barbara Calamini, Donald Lo and Judith Frydman will be at 10:30 a.m. on Wednesday, February 19, 2014 in Hall D in San Francisco's Moscone Convention Center. Abstract: tinyurl.com/or3apns