Trimethoprim antibiotic more effective against streptococci than expected
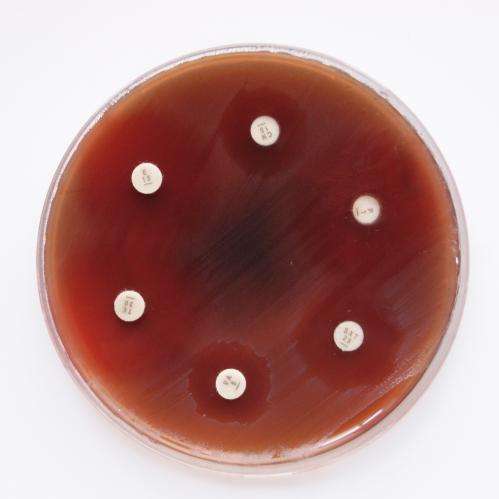
In less-developed countries, inexpensive and well-tolerated antibiotics for therapy of streptococcal infections are often not available. Scientists of the HZI in Germany have discovered that trimethoprim may provide an option. Contrary to a long-held belief, the bacteria are not generally resistant to this agent. In their latest publication the scientists demonstrated three pathways for the development of resistance—meaning that streptococci can easily become resistant to the antibiotic and pass on this trait quickly.
The common bacterium Streptococcus pyogenes is responsible not only for scarlet fever, a childhood disease presenting with characteristic skin rash, but also for many suppurative infections of the skin. The infection can be associated with serious consequences such as acute rheumatic fever and inflammation of the kidneys. In Germany, physicians usually prescribe penicillin, an antibiotic. In less-developed countries, penicillin is not always an option though. Firstly, penicillin is often not available and secondly, co-infections, i.e. concurrent infections, by another bacterium called Staphylococcus aureus occur and this microorganism is often no longer susceptible to the action of penicillin.
A group of scientists directed by Dr Patric Nitsche-Schmitz of the HZI entered into cooperation with the German National Reference Center for Streptococci in Aachen, Germany, to investigate if the antibiotic trimethoprim can be helpful in these scenarios. Trimethoprim inhibits an enzyme of folic acid metabolism called dihydrofolate reductase, which plays an important role in bacterial growth. Trimethoprim thus prevents bacteria from proliferating in the body. In the past, doctors advised against the use of this medication for treatment of streptococcal infections. The underlying reasoning was the wide-spread belief that the bacteria are already resistant to this agent, a misconception, as is becoming increasingly more evident. The reason for this mistake is that early studies used a culture medium that reduces the anti-microbial effect of trimethoprim.
The scientists from Braunschweig investigated samples from infected patients from Germany and India for resistance to trimethoprim. The majority of the samples were sensitive to the agent. "This shows that trimethoprim is indeed effective in many cases of Streptococcus pyogenes infection," Nitsche-Schmitz said.
The focus of his team was also on samples, in which the bacteria failed to respond to the agent. They discovered two types of resistance. "Spontaneous mutations can occur in the gene for dihydrofolate reductase rendering trimethoprim no longer able to attack the changed enzyme, which means it becomes ineffective," Nitsche-Schmitz explained. The team from Braunschweig detected a specific mutation in this gene in many samples, which renders streptococci resistant. In addition, bacteria can transfer copies of changed variants of the dihydrofolate reductase gene to other bacteria. This process called horizontal gene transfer allows resistance to spread very rapidly. The scientists found two genes of this type to be further causes of insensitivity.
The study shows that the antibiotic trimethoprim is a therapeutic option for Streptococcus pyogenes infections in some geographical regions of the world. The frequency of resistance is much lower than previously believed and the medication is inexpensive, stable and effective in Staphylococcus aureus co-infections. "However, it is like a sword that can loose its sharpness quickly," Nitsche-Schmitz said. "We found three causes for the rapid spread of resistance. It is important that trimethoprim, like all antibiotics, is not prescribed without need and that patients take the agent in accordance with the instructions given."
More information: René Bergmann, Mark van der Linden, Gursharan S. Chhatwal and D. Patric Nitsche-Schmitz, Factors that cause trimethoprim resistance in Streptococcus pyogenes, Antimicrobial Agent and Chemotherapy, 2014, DOI: 10.1128/AAC.02282-13