Drug watchdog urges vigilance in cancer drug theft
Europe's medicine watchdog urged doctors Thursday to be vigilant in administering the cancer drug Herceptin, vials of which had been stolen in Italy and tampered with before being sold back into the supply chain.
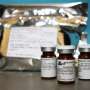
Vials of the drug had been stolen from Italian hospitals in several incidents since last December, and some were tampered with before being resold to wholesalers, said the European Medicines Agency.
It said "only a small number of vials" found their way back into the market in Europe, but could not say how many. And the agency stressed there was no evidence of any harm having come to patients.
Vials of another cancer drug, Alimta and of Remicade, an anti-inflammatory, have also been stolen, but have not yet been found back in circulation, the agency added.
"Healthcare professionals are reminded to pay extra attention when handling or administering any of the concerned medicines," it said in a statement.
Fake Herceptin vials can be identified by batch numbers and expiry dates on vials not matching the outer package, the presence of a liquid in vials meant to contain a powder, or evidence of tampering on the rubber stoppers.
Herceptin, manufactured by Swiss drug giant Roche, is used to treat breast and stomach cancer.
© 2014 AFP