Early chemotherapy improves survival for men with prostate cancer
Two papers from UCL show that having early chemotherapy improves survival for men with prostate cancer. The papers, published in the Lancet and Lancet Oncology, report the results from the STAMPEDE clinical trial and a meta-analysis.
Both papers looked at the use of a chemotherapy drug called docetaxel. Docetaxel is already used for men with prostate cancer once hormone therapy has stopped working. In STAMPEDE and the meta-analysis, the researchers looked at using it earlier, when men are starting long-term hormone therapy. Both studies found that adding docetaxel improved survival for these men.
The studies also looked at whether the drug zoledronic acid improves survival. Zoledronic acid is used to reduce the risk of bone problems in men whose cancer has spread to their bones, and whose hormone therapy has stopped working. The new studies looked at using it earlier, when men are starting long-term hormone therapy. Both studies found that adding zoledronic acid did not improve survival for these men.
STAMPEDE trial results
The STAMPEDE prostate cancer trial:docetaxel and zoledronic acid results for patients from MRC Clinical Trials Unit at UCL on Vimeo.
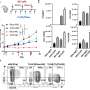
Men in the group given the standard treatment plus docetaxel lived on average for 10 months longer than men who had the standard treatment alone.
Men in the group given the standard treatment plus zoledronic acid did not live longer, on average, than men who had the standard treatment alone.
Men in the group given the standard treatment plus docetaxel plus zoledronic acid lived longer, on average, than those who had just the standard treatment. However, adding zoledronic acid to docetaxel did not seem to add any benefits beyond just docetaxel and the standard treatment.
Many men already report side-effects from the standard treatment, but men who had docetaxel were more likely to experience unwanted side‑effects compared to men on the standard treatment alone. The most common extra side-effects men on docetaxel reported were a low number of white blood cells, which increases the risk of infection. However, these side-effects were manageable and were generally short-term. After one year, there was no difference in the numbers of severe side-effects reported by men in any of the trial arms. Very few patients had to stop docetaxel due to side-effects.
The STAMPEDE researchers say that docetaxel should be routine practice in men with newly-diagnosed prostate cancer that has spread to other parts of the body if they are well enough to take it. Doctors should also consider it for men with high-risk prostate cancer that has not spread to other parts of the body, as it delays the disease getting worse.
Meta-analysis results
STAMPEDE results on docetaxel and zoledronic acid: summary for health workers from MRC Clinical Trials Unit at UCL on Vimeo.
The meta-analysis brought together the results of STAMPEDE and all similar trials that were available. It found that, in men whose disease had spread to distant parts of their body, docetaxel increased the proportion of men alive at four years after joining the trial from 40% to 49%. It also increased the proportion of men whose cancer had not come back or got worse, from 20% to 36% after four years.
For men whose disease had not yet spread to distant parts of their body, docetaxel increased the proportion whose cancer had not come back or got worse after four years. However, there was no clear evidence yet that it helped men to live for longer. Further evidence will emerge as more trial results are published and trials like STAMPEDE follow-up patients for longer.
The meta-analysis found no evidence that zoledronic acid helped men to live for longer.
More information: Nicholas D James et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial, The Lancet (2015). DOI: 10.1016/S0140-6736(15)01037-5
Claire L Vale et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data, The Lancet Oncology (2015). DOI: 10.1016/S1470-2045(15)00489-1