'Miracle' weight-loss drugs could have reduced health disparities: Instead they got worse
The American Heart Association calls them "game changers."
Apr 22, 2024
0
1
The American Heart Association calls them "game changers."
Apr 22, 2024
0
1

Metachromatic leukodystrophy is a rare genetic disorder that mainly affects young children and results in severe neurological symptoms accompanied by a loss of motor and intellectual capacities. At Paris Brain Institute, ...
Apr 22, 2024
0
8

European researchers are pioneering a vaccine and treatment for amyotrophic lateral sclerosis.
Apr 19, 2024
0
12
With more than 200 types of cancer and every cancer individually unique, ongoing efforts to develop precision oncology treatments remain daunting. Most of the focus has been on developing genetic sequencing assays or analyses ...
Apr 18, 2024
0
50

New evidence-based research calls into question the conventional three-month blanking period immediately after atrial fibrillation (AF) ablation when early occurrences of AF are thought not to predict long-term AF recurrence. ...
Apr 17, 2024
0
1

It's long been recognized that some of the groups most likely to get dementia, including African Americans and Hispanics, are greatly underrepresented in clinical trials. Now a new USC study shows that people from certain ...
Apr 17, 2024
0
55
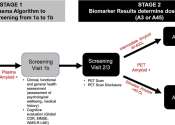
The interplay between the food we eat and our overall cognitive performance is a topic of increasing interest to people throughout the world. And while some studies have reported benefits of healthy diet patterns on cognitive ...
Apr 17, 2024
0
5

Through millions of years of evolutionary refinement, the human body has developed a sophisticated surveillance mechanism—the immune system. This intricate network is constantly scanning the body for invaders such as bacteria, ...
Apr 17, 2024
0
1

A comparison of treatments for malnutrition enteropathy, caused by severe acute malnutrition (SAM), has found evidence supporting the use of treatments to enhance the healing of mucosal membranes and reduce inflammation in ...
Apr 17, 2024
0
27

Researchers have used artificial intelligence techniques to massively accelerate the search for Parkinson's disease treatments.
Apr 17, 2024
0
77
