Newts which Regrow their Hearts

When a newt loses a limb, the limb regrows. What is more, a newt can also completely repair damage to its heart. Scientists at the Max Planck Institute for Heart and Lung Research in Bad Nauheim have now started to decode the cellular mechanisms in this impressive ability to regenerate and have discovered the remarkable plasticity of newt heart cells. As mammals, and therefore also humans, do not have this ability, the findings could contribute to new cell therapies for patients with damaged organs (Journal of Cell Science, 2006).
The red-spotted newt, Notophthalmus viridescens, is a favourite animal of the researchers working with Thomas Braun in Nauheim. This amphibian comes from the wetlands of North America, but it also feels quite at home in the Institute’s aquaria. It is a small animal that scientists find interesting for a particular reason: whereas humans cannot regenerate damaged heart muscle adequately after a heart attack and the destroyed muscle tissue scars over instead, following damage, a newt’s heart can be completely repaired and the organ’s function can be completely restored.
The key to this ability to regenerate are the heart muscle cells themselves. When a newt’s heart sustains damage, its cells can lose their characteristic properties; they can dedifferentiate. The researchers were able to show that proteins typical of heart muscle cells - the heavy myosin chain and various troponins - were dramatically down-regulated in this process. At the same time, the cells embark on massive cell division to build up new heart muscle. It takes around two weeks for the heart function to be restored in the newt. The data shows that at this point the expression of the muscle-specific proteins is again normal, i.e. the cells have differentiated again, and have regained their characteristic properties.
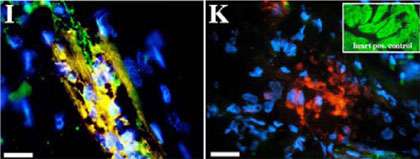
The researchers isolated the heart muscle cells and cultured them. In most of the cells, Braun and his colleagues were able to demonstrate the existence of a protein called Phospho-H3. This protein is a marker for the G2 phase of the cell cycle and indicates that the newt heart regenerates without the involvement of stem cells. It also seems that the heart regeneration does not create typical wound healing tissue, called a blastema. Braun explains this finding: "The heart only has a relatively small number of different cell types. This could be a reason why the regeneration of heart tissue does not require a blastema." The researchers in Bad Nauheim found no indication that stem cells were involved in repairing newt hearts.
The process of regenerating lost extremities is different. Unlike in the process with the heart, newts develop a blastema in this case. Blastema cells have certain characteristics in common with stem cells, such as the development into different cell types. The cell biologists in Bad Nauheim injected isolated heart muscle cells into a newt’s leg that was regrowing after amputation. In this environment, the cells began to de-differentiate, as they did in the heart. However, this did not happen when they were injected into an undamaged extremity. Again, the researchers registered the very rapid loss of heart muscle-specific proteins.
"We suspect that the signal for the de-differentiation comes from the area where the wound is healing and the cells communicate with each other," explains Braun. These signals could be transmitted via certain enzymes, for example. An enzyme of this nature - focal adhesion kinase -, which plays a part in the transmission of signals in the cells, is phosphorylated in the transplanted cells and is thus active. The Max Planck researchers in Bad Nauheim hope that better understanding of the molecular issues involved in regeneration in the newt will open up new possibilities for the repairing human patients’ damaged hearts.
Citation: Friedemann Laube, Matthias Heister, Christian Scholz, Thilo Borchardt, Thomas Braun, Re-programming of newt cardiomyocytes is induced by tissue regeneration, Journal of Cell Science, 119 (22), 2006
Source: Max-Planck-Institute for Heart and Lung Research