Dominant cholesterol-metabolism ideas challenged by new research
A team of researchers investigating cholesterol and lipid transport has performed experiments that cast serious doubt on the dominant hypothesis of how the body rids its cells of "bad" cholesterol (LDL) and increases "good" cholesterol (HDL). Cholesterol metabolism is an area of intense inquiry because high levels of LDL cholesterol or total cholesterol put about half of all Americans at significant risk of heart disease.
The team was led by Robert A. Schlegel, Professor of Biochemistry and Molecular Biology at Penn State University, and Patrick Williamson, the Edward S. Harkness Professor of Biology at Amherst College. The paper by Schlegel, Williamson, and their colleagues will be published on Wednesday, 15 August 2007, in the on-line journal PLoS One.
A protein called ABCA1 is critical for producing "good" cholesterol: patients who lack the gene for this protein produce no HDL, and as a result, suffer from heart attacks at an early age. An important question is what ABCA1 does that is so important for producing HDL.
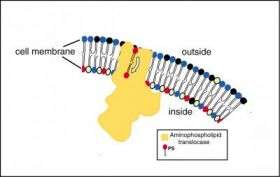
The most popular hypothesis was put forth in 2000 by Giovanna Chimini, Group Leader of a laboratory in the Centre d'Immunologie Marseille-Luminy in France, and colleagues. They suggested that the ABCA1 enzyme plays a major role in the transfer of a phospholipid called phosphatidylserine (PS) from the inside of the cell -- where it is normally concentrated -- across the cell membrane to the outer surface of the cell. This is a crucial first step in the mechanism by which excess lipids and cholesterol are eliminated from the body. Under normal conditions, there is a second important step. After the PS is expressed on the cell surface, the phospholipids and excess cholesterol can be loaded onto a circulating protein, apoA1, to generate an HDL cholesterol particle. HDL cholesterol carries the lipids through the blood stream to the liver, where they are dumped into the intestine for excretion or destruction.
The expression of PS on the cell's surface might be accomplished in one of two ways. First, the enzyme that transports PS into the cell -- aminophospholipid translocase -- might be inhibited or blocked by ABCA1, causing a passive build-up of PS on the cell surface. Alternatively, ABCA1 might activate or might actually be the enzyme, scramblase, which causes PS and other phospholipids to redistribute until there are equal amounts inside and outside the cell membrane, making it possible to load the phospholipids and cholesterol onto apoA1.
During programmed cell death (apoptosis), PS is also expressed on the surface of apoptotic cells to signal macrophages to come and clean up the dead cells. Both the macrophages and apoptotic cells must express PS on their surfaces for this mechanism to work normally. Therefore, Chimini also proposed that ABCA1 might be part of the molecular machinery involved in apoptosis and macrophage activity.
The Penn State-Amherst team performed a complex series of experiments to determine whether the ABCA1 enzyme is either actively involved in the transmembrane transport of phospholipids or regulates another enzyme that transports the phospholipids. They also examined the role of ABCA1 enzyme in apoptosis and macrophage activity.
A key part of the study was using cells from sufferers of a rare genetic disorder, Tangier disease, who lack the ABCA1 gene. For comparison, the team also studied cells from experimental mice in which the gene for ABCA1 has been knocked out.
Exacting measurements of the movement of various phospholipids across the membrane of normal cells showed that the rate of phospholipid transport into the cell is not affected by the presence or absence of the ABCA1 gene and its corresponding enzyme. Similar experiments with Tangier cells or knock-out mouse cells produced the same results. "This showed us," says Schlegel, "that the ABCA1 enzyme was not involved in the transport of phospholipids across the cell membrane under normal conditions."
The team then made use of the fact that the scramblase can be activated in some cells in the laboratory by increasing the concentration of calcium ions. This activation mechanism works best in red blood cells, platelets, lymphocytes, and macrophages and does not work at measurable levels in fibroblasts or epithelial cells. In cells activated by calcium ions, neither the presence nor the absence of ABCA1 changes phospholipids transport. In cells that cannot be activated by adding calcium ions, adding ABCA1 as well does not induce activation. The experiments have the same outcome whether the team used cells from Tangier individuals or knock-out mice. These results suggest strongly that ABCA1 does not code for scramblase and is not required for its activation.
The team then turned to the function of ABC1 in apoptosis. Did ABCA1 cause or activate phospholipid transport across the cell membrane only during apoptosis? "We could not demonstrate that the addition or deletion of ABCA1 had any effect on the movement of PS to the cell surface in apoptotic cells," says Schlegel. "We conclude that the ABCA1 enzyme doesn't influence the transport of PS either under normal conditions or in apoptotic cells."
A third possibility was that ABCA1 functioned only in macrophages to cause the expression of PS on the cell surface. Additional experiments refuted this suggestion too. The absence of ABCA1 did not prevent macrophages from expressing PS on their surfaces. Further, the rate at which macrophages recognized and engulfed their target apoptotic cells remained constant whether ABCA1 was present in the macrophages or not.
"This work really changes our understanding of the mechanics of cholesterol metabolism," remarks Schlegel. "We can't find any evidence whatsoever to support the popular hypothesis that the ABCA1enzyme catalyzes the movement of PS across the cell membrane to the outer surface of the cell. Yet because Tangier patients and the knock-out mice experience a serious build-up of LDL in the tissues, we know that ABCA1 plays an important role in the cholesterol metabolism. Since none of our experimental results were consistent with the hypothesis that ABCA1 influences the first step in the process -- the transport of phospholipids to the cell surface -- we think that ABCA1 must operate in the second step, when the phospholipids are loaded onto the apoA1."
Source: Penn State





















