Research shows skeleton to be endocrine organ
Bones are typically thought of as calcified, inert structures, but researchers at Columbia University Medical Center have now identified a surprising and critically important novel function of the skeleton. They’ve shown for the first time that the skeleton is an endocrine organ that helps control our sugar metabolism and weight and, as such, is a major determinant of the development of type 2 diabetes.
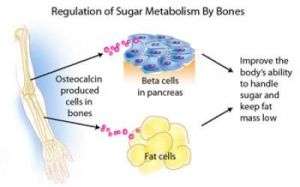
The research, published in the August 10 issue of Cell, demonstrates that bone cells release a hormone called osteocalcin, which controls the regulation of blood sugar (glucose) and fat deposition through synergistic mechanisms previously not recognized. Usually, an increase in insulin secretion is accompanied by a decrease in insulin sensitivity. Osteocalcin, however, increases both the secretion and sensitivity of insulin, in addition to boosting the number of insulin-producing cells and reducing stores of fat.
In this published research, authors show that an increase in osteocalcin activity prevents the development of type 2 diabetes and obesity in mice. This discovery potentially opens the door for novel therapeutic avenues for the prevention and treatment of type 2 diabetes.
“The discovery that our bones are responsible for regulating blood sugar in ways that were not known before completely changes our understanding of the function of the skeleton and uncovers a crucial aspect of energy metabolism,” said Gerard Karsenty, M.D., Ph.D., chair of the department of Genetics and Development at Columbia University Medical Center, Paul Marks Professor in the Basic Sciences, and senior author of the paper. “These results uncover an important aspect of endocrinology that was unappreciated until now.”
Karsenty and his colleagues had previously shown that leptin, a hormone released by fat cells, acts upon and ultimately controls bone mass. They reasoned that bones must in turn communicate with fat, so they searched bone-forming cells for molecules that could potentially send signals back to fat cells.
The researchers found that osteocalcin, a protein made only by bone-forming cells (osteoblasts), was not a mere structural protein, but rather a hormone with totally unanticipated and crucial functions. Osteocalcin directs the pancreas’ beta cells, which produce the body’s supply of insulin, to produce more insulin. At the same time, osteocalcin directs fat cells to release a hormone called adiponectin, which improves insulin sensitivity. This discovery showed for the first time that one hormone has a synergistic function in regulating insulin secretion and insulin sensitivity, and that this coordinating signal comes from the skeleton. Additionally, osteocalcin enhances the production of insulin-producing beta cells, which is considered one of the best, but currently unattainable, strategies to treat diabetes.
People with type 2 diabetes have been shown to have low osteocalcin levels, suggesting that altering the activity of this molecule could be an effective therapy. That hypothesis is supported by the Columbia research, which showed that mice with high levels of osteocalcin activity were prevented from gaining weight or becoming diabetic even when they ate a high fat diet. Analysis of mice lacking the osteocalcin protein showed that they had type 2 diabetes, increased fat mass, a decrease in insulin and adiponectin expression, and decreased beta-cell proliferation.
This research was supported by the National Institutes of Health, the American Diabetes Association, the Japan Society for the Promotion of Science, and the Pennsylvania Department of Health.
The researchers are now examining the role of osteocalcin in the regulation of blood sugar in humans and are continuing investigations into the relationship between osteocalcin and the appearance of type 2 diabetes and obesity.
Source: Columbia University Medical Center