New study reveals for first time how BRCA1 mutations cause breast cancer
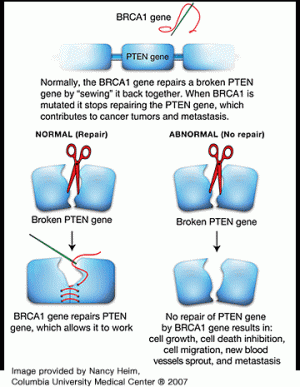
An international team of researchers led by Columbia University Medical Center’s Herbert Irving Comprehensive Cancer Center and Sweden’s Lund University has, for the first time, revealed how mutations in the BRCA1 gene lead to breast cancer. Findings show that one way BRCA1 mutations cause cancer is by knocking out a powerful tumor suppressor gene known as PTEN.
The new study will be published online on the Nature Genetics website on Dec. 9, 2007: http://www.nature.com/ng. It will appear in the January print issue of this journal. The study was led by Ramon Parsons, M.D., Ph.D., the Avon Foundation Professor of Medicine and Pathology at Columbia University College of Physicians and Surgeons and Åke Borg, Ph.D., professor of oncology at Lund University. The paper’s first author was Lao Saal, Ph.D. (now finishing his medical degree at Columbia University College of Physicians and Surgeons).
“These findings are exciting because ever since the link was established between BRCA1 and breast cancer more than 10 years ago, we have been frustrated by our lack of understanding about how mutations in this gene cause breast cancer. We have been stymied by our limited resources to treat these cancers, which are associated with very poor prognoses. Now that we know that PTEN is involved, we finally have a target for therapy for these cancers,” said Dr. Parsons, the study’s corresponding author. Dr. Parsons is director of the Avon Foundation Breast Cancer Research Laboratory and director of the Breast Cancer Program of the Herbert Irving Comprehensive Cancer Center at Columbia University Medical Center and NewYork-Presbyterian Hospital.
In 1997, Dr. Parsons led one of the two teams that independently discovered the PTEN, one of the most important tumor suppressor genes altered in breast cancer, as well as in brain and prostate cancers. PTEN is now recognized to be mutated in about 30 percent of all cancers, making it the second most mutated gene in cancer after p53. Knocking out PTEN sends a strong pro-growth signal on tumor cells. This is unlike the BRCA1 mutation, which only predisposes the cells to accumulate genetic damage and sends an indirect signal for cell growth. “Once a cell loses PTEN, it has a growth advantage over its neighbors and starts on the road to cancer,” said Dr. Parsons.
PTEN mutations promote runaway tumor cell growth by increasing the activity of a series of different proteins in the cell known as the PTEN/PI3K pathway. Shutting down any one of those proteins could potentially stop growth of the cancer. Investigational therapies to shut down proteins in the PTEN pathway are currently in Phase I clinical trials.
How the BRCA1 Mutation Mechanism Was Pinpointed
Dr. Parsons and his research team made the connection between BRCA1 and PTEN using techniques to search for physical chromosome breaks within the PTEN gene – a technique that had never before been used. Previous searches for PTEN mutations in BRCA1 tumors had looked for conventional mutations and failed to turn up any abnormalities.
The researchers scanned 34 biopsies taken from women with BRCA1 tumors. The PTEN gene had been split in two, but inadequately repaired in about one-third of the cancers. In some cases, entire sections of the gene were missing; in others, one-half of the gene was reattached to other regions on the chromosome.
These types of large chromosomal mistakes stem directly from the tumor’s lack of BRCA1, a gene that is normally involved in the repair of such damage. In breast cancers from women with normal BRCA1, such large mutations in PTEN were rarely detected.
Finding May Affect 50% of BRCA1 Breast Cancers & Lead to New Treatments
Dr. Parsons estimates that about 50 percent of BRCA1 breast cancers will be found to harbor mutated PTEN once a complete analysis of chromosomal mutations is done.
Breast cancer tumors caused by BRCA1 are known as basal-like or triple-negative because these tumors usually lack estrogen, progesterone, and HER2 receptors, which are needed for most breast cancer treatments to be effective. Basal-like breast tumors are found in 10 to 20 percent of women with non-hereditary breast cancer (meaning, not caused by a genetic mutation in BRCA1 or another gene), and the researchers found that PTEN is also lost in the majority of these breast tumors as well.
“Our results point to PTEN as a major player in both hereditary and non-hereditary basal-like breast cancer, a finding that may now be exploited to develop new therapeutic strategies to improve outcomes for women with these aggressive tumors,” said Dr. Saal, who at the time of the research, was a fellow in Dr. Parsons’ Avon Foundation Breast Cancer Research Laboratory.
The researchers also predict that other cancer genes besides PTEN are targeted by BRCA1. “By using the same techniques we used to find gross chromosomal rearrangements in PTEN, we hope to start identifying additional mutated genes involved in the development of breast cancer,” said Dr. Parsons.
“These kinds of mutations that break tumor suppressors in half may turn out to be common in many kinds of carcinomas, particularly those with deficiencies in DNA repair pathways similar to BRCA1, a question that only a systematic search can answer,” said Dr. Saal.
“Similar research is underway in tumors from carriers of germline mutations in BRCA2, the other known major breast cancer susceptibility gene,” said Dr. Borg. “BRCA2 has a role downstream in the same DNA double strand break repair pathway as BRCA1, but tumors from BRCA2 mutation carriers have a quite different phenotype compared to BRCA1 tumors, less often involving PTEN loss. However, like BRCA1, BRCA2 tumors have an instable genome with massive chromosomal aberrations, suggesting that other genes may be targeted.”
Breast Cancers Caused by BRCA1 Mutations are Especially Lethal & Difficult to Treat
Basal-like breast cancer tumors, whether caused by BRCA1 mutations or of the non-hereditary type, are among the most aggressive tumors – they grow fast and spread quickly, making them more likely than other types of cancer to be fatal. These tumors are more likely to be resistant to standard breast cancer treatments, such as Tamoxifen or Herceptin, making them especially difficult to treat. As a result, many young BRCA1 carriers opt to have their breasts prophylactically removed instead of waiting for cancer to appear.
Breast cancers caused by BRCA1 mutations tend to affect women much earlier – often before menopause and sometimes in their 20s and 30s – and between 60 and 80 percent of women who carry a BRCA1 mutation will develop breast cancer at some point during their lives. BRCA1 mutation carriers are most common among African-American women and women of Ashkenazi Jewish descent. Inherited BRCA1 (and BRCA2) mutations also predispose women to ovarian cancer, a disease that frequently escape early diagnosis and which has a fatal outcome in advanced stages.
International Collaborations & Research Support
Additional Columbia researchers involved in the study include Hanina Hibshoosh, M.D., associate professor of clinical pathology and Vundavalli Murty, M.D., associate professor of pathology, and others.
“Identifying these rearrangements would not have been possible without the support of the shared resources of the Herbert Irving Comprehensive Cancer Center and our collaboration with Drs. Borg, Saal, Hibshoosh and Murty,” said Dr. Parsons.
Source: Columbia University