How small molecule can take apart Alzheimer's disease protein fibers
Researchers from the University of Pennsylvania School of Medicine have shown, in unprecedented detail, how a small molecule is able to selectively take apart abnormally folded protein fibers connected to Alzheimer's disease and prion diseases. The findings appear online this week in the Proceedings of the National Academy of Sciences. Finding a way to dismantle misfolded proteins has implications for new treatments for a host of neurodegenerative diseases.
Abnormal accumulation of amyloid fibers and other misfolded forms in the brain cause neurodegenerative diseases. Similarly, build-up of abnormally folded prion proteins between neurons causes the human version of mad cow disease, Creutzfeldt-Jakob disease.
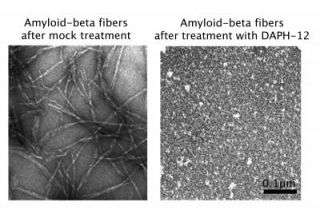
“Surprisingly, a small molecule called DAPH selectively targets the areas that hold fibers together, and converts fibers to a form that is unable to grow. Normally fibers grow from their ends, but the drug stops this activity,” says senior author James Shorter, PhD, Assistant Professor of Biochemistry and Biophysics. “Our data suggest that it is possible to generate effective small molecules that can attack amyloid fibers, which are associated with so many devastating diseases.”
The researchers are now working on how DAPH acts as a wedge to stop the fibers from growing. “Presumably DAPH fits very neatly into the crevices between fiber subunits,” explains Shorter. “When we grow yeast cells with the prion in the presence of DAPH, they begin to lose the prion. We also saw this in the test tube using pure fibers. The small molecule directly remodels fiber architecture. We’ve really been able to get at the mechanism by which DAPH, or any small molecule, works for the first time.” DAPH was originally found in a screen of small molecules that reduce amyloid-beta toxicity in the lab of co-author Vernon Ingram, Shorter’s collaborator at the Massachusetts Institute of Technology (MIT).
In a test tube, if a small amount of amyloid or prion fiber is added to the normal form of the protein, it converts it to the fiber form. But when DPAH is added to the mix, the yeast prion protein does not aggregate into fibers. “It’s essentially stopping fiber formation in its tracks,” says Huan Wang, first author and research specialist in Shorter’s lab. “We were surprised to see two very different proteins, amyloid-beta and Sup35, sensitive to this same small molecule.”
The next step is to identify more potent DAPH variants with greater selectivity for deleterious amyloids. Since some amyloids may turn out to be beneficial – for example, one form may be involved in long-term memory formation – it will be necessary to find a drug that does not hit all amyloids indiscriminately. “We’d need one that hits only problem amyloids, and DAPH gives us a hint that such selectivity is possible” says Shorter.
Source: University of Pennsylvania