Safety study indicates gene therapy for blindness improves vision
All three people who received gene therapy at the University of Florida to treat a rare, incurable form of blindness have regained some of their vision, according to a paper published online today in Human Gene Therapy.
The patients — one woman and two men ranging from 21 to 24 years old with a type of hereditary blindness called Leber congenital amaurosis type 2 — volunteered to test the safety of an experimental gene-transfer technique in a phase 1 clinical research study conducted by UF and the University of Pennsylvania with support from the National Eye Institute of the National Institutes of Health.
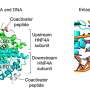
In this form of LCA disease, photoreceptor cells cannot respond to light because a gene called RPE65 does not properly produce a protein necessary for healthy vision. In the study, researchers used an adeno-associated virus — an apparently harmless virus that already exists in most people — to deliver RPE65 to a small area of the retina.
Not only were there no ill effects other than routine postsurgical soreness, the subjects said the vision in their treated eyes was slightly improved in dim lighting conditions.
"The patients report seeing brighter areas and perhaps some images, but basically the message is that this is treatment is fully safe," said William W. Hauswirth, Ph.D., a professor of ophthalmology and member of UF's Powell Gene Therapy Center and the UF Genetics Institute.
"One thing we did not do is suppress the patients' immune systems, which was done in two other LCA clinical trials that were under way," said Hauswirth, who began studying the adeno-associated virus as a vehicle to deliver genes into living animals more than 30 years ago. "Theoretically, the idea was that it might be necessary to suppress the immune system because we are using a vector that might activate the body's defenses and cause a harmful response. However, immune suppression itself carries a risk of infections and other problems. Clearly we have shown there is no need to do that in this case."
Samuel G. Jacobson, M.D., Ph.D., a professor of ophthalmology with the Scheie Eye Institute at the University of Pennsylvania, is the study's principal investigator.
"This groundbreaking gene therapy trial builds on 15 years of research sponsored by the National Eye Institute of NIH," said Paul A. Sieving, M.D., Ph.D., director of the NEI. "The study has partially restored vision in three young adults, and it demonstrates that gene therapy can be effective in treating human vision disease. Many human diseases are inherited in families and result from mutations in single genes. These genetic conditions are particularly suited to potential treatment by gene therapy. This trial to treat vision loss from the condition of Leber congenital amaurosis is an important demonstration of proof of principle and shows that we are on the right track. We can now invest in further work to refine, and ultimately to expand, genetic treatment approaches."
Results published today focus on the health of the entire retina, not just the tiny portion that received the gene therapy. A detailed examination of the therapy's effectiveness in the treated portion of the eye will appear in an upcoming issue of the Proceedings of the National Academy of Sciences. Two other recent LCA clinical trial reports appeared recently in The New England Journal of Medicine.
"The safety study itself is a milestone, but when we see a benefit to the subject — that is a truly a welcome bonus," said Barry J. Byrne, M.D., Ph.D., a professor of molecular genetics and microbiology and director of UF's Powell Gene Therapy Center, which manufactured the viral vectors used in the study. "Improvements in someone's medical condition are ultimately what we are after."
LCA2 affects about 2,000 people in the United States and is one of several incurable forms of blindness collectively known as retinitis pigmentosa, which in turn affects about 200,000 Americans.
Children with LCA2 experience major visual disability that can lead to total vision loss in adulthood. Although vision loss is severe, the structure of the retina — including its connection to the brain — can remain relatively intact for decades before the photoreceptor cells degenerate.
Source: University of Florida