First genetic link between reptile and human heart evolution
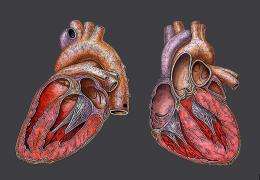
Scientists at the Gladstone Institute of Cardiovascular Disease have traced the evolution of the four-chambered human heart to a common genetic factor linked to the development of hearts in turtles and other reptiles.
The research, published in the September 3 issue of the journal Nature, shows how a specific protein that turns on genes is involved in heart formation in turtles, lizards and humans.
"This is the first genetic link to the evolution of two, rather than one, pumping chamber in the heart, which is a key event in the evolution of becoming warm-blooded," said Gladstone investigator Benoit Bruneau, PhD, who led the study. "The gene involved, Tbx5, is also implicated in human congenital heart disease, so our results also bring insight into human disease."
From an evolutionary standpoint, the reptiles occupy a critical point in heart evolution.
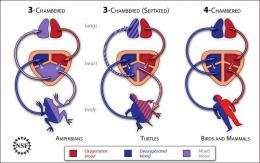
While bird and mammalian hearts have four chambers, frogs and other amphibians have three. "How did hearts evolve from three to four chambers?" Bruneau said. "The different reptiles offer a sort of continuum from three to four chambers. By examining them, we learned a lot about how the human heart chambers normally form."
He explained that with four chambers—two atria and two ventricles—humans and all other mammals have completely separate blood flows to the lungs and to the rest of the body, which is essential for us to be warm-blooded.

When it comes to reptiles, such as turtles and lizards, there is debate about whether they have one or two ventricles, which are the pumping chambers. "The main question for us to understand the evolution of the heart was to identify the true nature of these early reptile ventricles and to figure out what controls the separation of the heart into left and right sides," said Dr. Bruneau.
To better understand reptile heart evolution, Dr. Bruneau's team used modern molecular genetics to examine Tbx5. Mutations in the human gene that encodes Tbx5 result in congenital heart disease and, in particular, defects in the ventricular septum, the muscular wall that separates the ventricle into two sections. Tbx5 is a transcription factor, a protein that turns other genes on or off. In humans and other mammals, Tbx5 levels are high in the left ventricle and low in the right. The boundary of high and low levels is right where the septum forms to divide the ventricle into two parts. "Based on these observations," said Dr. Bruneau, "we thought Tbx5 was a good candidate as a key player in the evolution of septation."
The team looked at Tbx5 distribution in the turtle and the green anole lizard. During the early stages of heart formation in both reptiles, Tbx5 activity is found throughout the embryonic ventricular chamber. In the lizard, which forms only one ventricle, this pattern stays the same as the heart develops. However, in the turtle, which has a primitive septum that partially separates the ventricles into left and right sides, distribution of Tbx5 is later gradually restricted to the area of the left ventricle, resulting in a left-right gradient of Tbx5 activity. This meant that the gradient of Tbx5 forms later and less sharply in the turtle than in species with a clear septum, such as mammals, providing a tantalizing clue about how septation evolved.
They then wanted to determine whether Tbx5 was really a main regulator of septation or merely a bystander. Mice were genetically engineered to express Tbx5 at a moderate level throughout the developing heart, just like in turtle hearts. By mimicking the turtle pattern, mouse hearts now resembled turtle hearts. The offspring from these mice died young and had only a single ventricle. This striking result conclusively showed that a sharp line delineating an area of high level of Tbx5 is critical to induce formation of a septum between the two ventricles.
"This really nailed the importance of Tbx5 in patterning the heart to allow septation to occur," said Dr. Bruneau.
During evolution, new genetic regulatory elements evolved to tell the Tbx5 gene to form a sharp boundary of Tbx5 expression. This resulted in two ventricles. Researchers will now work to identify those genetic regulatory mechanisms during the evolution of reptiles. The work also has important implications for the understanding of congenital heart defects, which are the most common human birth defect, occurring in one out of every one hundred births worldwide. Humans born with only one pumping chamber, resembling frog hearts, suffer the highest mortality and require extensive surgery as newborns.
"Our study provides exciting new insights into the evolution of the heart, which had not been examined in over 100 years," Dr. Bruneau explained. "In a larger context, it provides good support for the concept that changes in the expression levels of various regulatory molecules are important in evolution. From these studies we also hope to understand further how defects in septation occur in humans with congenital heart disease."
Source: Gladstone Institutes (news : web)















