Drug could provide first treatment for scleroderma
Investigators have identified a drug that is currently approved to treat certain types of cancer, Gleevec, that could provide the first treatment for scleroderma, a chronic connective tissue disease for which a treatment has remained elusive. The news will be presented at the annual meeting of the American College of Rheumatology on October 18 in Philadelphia.
"There has never been a drug that has been shown to be effective for this condition. I think there is a very good chance of Gleevec becoming a real treatment for a previously untreatable disease," said Robert Spiera, M.D., an associate attending rheumatologist at Hospital for Special Surgery who led the study.
For the study, investigators at Hospital for Special Surgery enrolled 30 patients with diffuse scleroderma, a widespread severe form of the disease, and gave them 400 mg of Gleevec per day. Patients were evaluated monthly for 12 months during treatment and were seen for follow-up three months after discontinuing the drug.
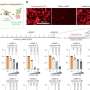
To measure the effectiveness of the drug, researchers used a tool known as the modified Rodnan skin score, a measure of how much skin is affected by the disease. "The skin score seems to be a very good marker of disease status and most scleroderma trials use this as an outcome measure," said Dr. Spiera, who is also an associate professor at Weill Cornell Medical College. The investigators also measured lung function using tests for forced vital capacity (FVC), the maximum volume of air that a person can exhale after maximum inhalation, and diffusion capacity, a measurement of the lung's capacity to transfer gases. Lung disease is the main cause of mortality in scleroderma.
The investigators reported an interim analysis of their results, although the study is ongoing. At one year, the investigators saw a 23 percent improvement in skin scores. The researchers also saw an improvement in forced vital capacity scores by 9.6 percent and diffusion capacity scores by 11 percent in the 18 patients who had completed one year of treatment.
"The lung function data was really exciting," Dr. Spiera said. "In patients with scleroderma, you usually see lung function tests getting worse over time, and if doctors try a therapy for a year and a patient doesn't get any worse, we get pretty excited. What is amazing to me in this study is that we actually saw improvements in both lung function tests."
The study is the largest single center trial of Gleevec in scleroderma to date with the longest duration of treatment and follow-up. Before this trial, test tube studies of human cells indicated that Gleevec might have some activity in combating the disease, and the drug was shown to be effective in a rodent model of the disease. Only anecdotal evidence, however, had been published on the drug's effectiveness in treating the disease in humans. Dr. Spiera said the findings of his open-label study need to be interpreted cautiously, and ultimately corroborated by evidence from a randomized controlled trial, the gold standard of clinical trials.
Until now, no drug has been shown to be effective in treating scleroderma in a clinical trial. Several years ago, a small study provided some evidence that a chemotherapy drug called cyclophosphamide may help scleroderma patients, but the benefit was minimal and this drug causes side effects including infertility and secondary cancers.
Dr. Spiera's study was funded primarily through the Rudolph Rupert Scleroderma Program at the Hospital for Special Surgery. Novartis, the manufacturer of Gleevec, provided some monetary support and donated drug. The company is not involved in the design or analysis of the trial. Gleevec is approved in the United States for two types of cancer: chronic myeloid leukemia and gastrointestinal stromal tumor.
Systemic scleroderma affects not only the skin, but also underlying blood vessels, and often muscles and joints, as well as the gastrointestinal tract, kidneys, lungs and heart. According to the Scleroderma Foundation, roughly 300,000 individuals have scleroderma in the United States and roughly a third of these have the systemic kind. The disease typically strikes in the prime of patients' lives, when they are 30-50 years old.
Source: Hospital for Special Surgery