Counterfeit drugs becoming big business worldwide
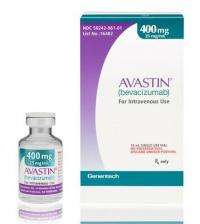
(AP) -- The discovery that a fake version of the widely used cancer medicine Avastin is circulating in the United States is raising new fears that the multibillion-dollar drug-counterfeiting trade is increasingly making inroads in the U.S.
The criminal practice has largely been relegated to poor countries with lax regulations. But with more medicines and drug ingredients for sale in the U.S. being manufactured overseas, American authorities are afraid more counterfeits will find their way into this country, putting patients' lives at risk.
The Avastin discovery follows other recent instances in the U.S. of counterfeiting, involving such drugs as Viagra, the cholesterol medicine Lipitor and the weight-loss pill Alli.
"We do know there are counterfeits continuing to try and make their way onto the U.S. supply chain," said Connie Jung, an associate director in the Food and Drug Administration's office of drug security.
The FDA announced Tuesday it is investigating fake vials of Avastin that were sold to at least 19 doctors and clinics, including 16 sites in California, two in Texas and one in Chicago. Tests showed the vials did not contain the active ingredient in Avastin, which is given intravenously in hospitals, clinics and doctors' offices to treat several types of cancer.
The contents of the vials are still being analyzed, and the FDA said it has not received any reports of patients who were harmed.
FDA officials said the counterfeit Avastin was imported from Britain and distributed by Volunteer Distribution, a wholesaler based in Gainesboro, Tenn. British regulators notified the FDA about the products in December, but the agency didn't confirm they were fake until last week.
The FDA gave assurances Wednesday that the U.S. remains one of the most secure pharmaceutical markets in the world. But the news sent cancer doctors scrambling to check their records.
Mary Mathias, a nurse who orders drugs for one doctor on the FDA list - Dr. Phillip L. Chatham in Granada Hills, Calif., - said they had stopped using the company in question at least a year ago.
Because Avastin treatments are spaced one to two weeks apart, it is not likely that someone would get more than one infusion from the same vial. And because these are people facing a life-threatening disease, it is hard to say whether missing one treatment with the real drug would compromise their care.
Gauging harm from a counterfeit cancer treatment is nearly impossible, said Dr. Robert C. Young, former president of the Fox Chase Cancer Center in Philadelphia and now a consultant to cancer centers.
A colon cancer patient, for example, might receive 18 to 20 Avastin infusions over six months. Missing one dose seems unlikely to have a dramatic effect on survival odds, but it's not provable either way because cancer's course and a patient's response to treatment are not predictable, he said.
Counterfeits have traditionally been more of a concern in developing regions like Asia and Latin America, where as many as 30 percent of drugs sold are fake, according to the World Health Organization. The group estimates just 1 percent of drugs dispensed in the U.S. and other developed nations are fake.
But incidents of counterfeiting reported by drugmakers have increased steadily over the decade to more than 1,700 worldwide last year, though only 6 percent of those were in the U.S. There are few reliable estimates on the value of the global counterfeit drug trade, though most place it in the tens of billions.
Counterfeiting has become more prevalent as pharmaceutical supply chains increasingly stretch across continents. Over 80 percent of the active ingredients used in U.S. pharmaceuticals are now manufactured overseas, according to a recent congressional report, and experts say this has made it easier to move counterfeit products into this country.
"With today's transportation networks, it's no longer a stretch to move these materials from a source in Pakistan or India to the U.S." said Tom Kubic, president of Pharmaceutical Security Institute, a trade association set up by two dozen pharmaceutical companies.
In 2005, federal prosecutors indicted 11 employees of a Missouri business on charges of conspiring to sell $42 million in counterfeit Lipitor. It was manufactured in Costa Rica and illegally imported to the U.S., where it was sold to wholesalers.
Industry experts also say a combination of big profits and low penalties has made drug counterfeiting an increasingly attractive business for criminals in the U.S. and abroad.
A single vial of Avastin sells for $2,400, and the drug had nearly $2.7 billion in U.S. sales last year, while the sentence for drug counterfeiting in the United States is about three years in prison. That compares with 15 years for counterfeiting money.
John Clark, head of global security for Pfizer Inc., said counterfeiters can make several million dollars quickly and, if they're caught, get off with as little as six months in jail. He also said counterfeiters can set up an operation at a fairly low cost - perhaps $50,000, including about $20,000 for a pill press.
"It's a no-brainer for criminal organizations that it's worth a gamble," Clark said.
Clark said Pfizer's anti-counterfeiting team around the world has seen a number of fake vaccines and biologic drugs sold in developing countries, not just pill-based drugs.
"They're getting much more sophisticated," often getting ahold of legitimate vials that had held such medicines, from patients, trash cans or recycling operations, and then filling them with oil or water.
©2012 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.















