New test helps evaluate cancer drug's merit
(HealthDay) -- A new genetic test to help doctors determine if the drug Erbitux would be an effective treatment for certain colorectal cancer patients has been approved by the U.S. Food and Drug Administration.
The KRAS RGQ PCR kit checks for the presence of a certain mutation in the KRAS gene. About 40 percent of people with colon cancer are thought to have the mutation, which leaves Erbitux ineffective. The test is for people whose colorectal cancer has spread to other parts of the body, the FDA said in a news release.
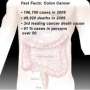
The American Cancer Society says more than 141,000 new cases of colon cancer were diagnosed last year in the United States, and nearly 50,000 people died of the disease.
Erbitux (cetuximab), co-marketed by Bristol-Myers Squibb and Eli Lilly, was first approved as a colon cancer treatment in 2004.
The new diagnostic was developed by Qiagen Manchester Ltd., based in Manchester, England.
More information: Visit the FDA to learn more about this approval.
Copyright © 2012 HealthDay. All rights reserved.