July 7, 2012 report
New detector for rare cancer cells
(Medical Xpress) -- Researchers in the US have developed a new detector for measuring rare circulating tumor cells (CTCs) in samples of whole blood.
The research team, led by Hakho Lee of the Massachusetts General Hospital developed a detector that uses the Hall effect, in which a voltage is produced when a magnetic field is applied perpendicular to the current running through a conductor. The Hall effect is often the basis of sensors and electronic compasses such as those used in global positioning systems (GPS).
Cells in the untreated samples were fed through channels on the chip on a micro-Hall Detector (μHD), where they were tagged with magnetic nanoparticles (MNPs). The prototype proved to be capable of processing 107 cells per minute.
The chip is a microfluidic/semiconductor hybrid with eight micro-Hall sensors. As the tagged cells pass through the chip they are subjected to a magnetic field, and the magnetic moment each acquires is sensed by at least two of the micro-Hall sensors. This arrangement of overlapping sensors means the channels can be wider and flow rates higher than if each cell was exposed to only one sensor.
The prototype’s ability to label and identify several different biomarkers on rare circulating cancer cells was first demonstrated, and then the system was tested on whole blood samples of 1micro leter volume, with cancer cells added. The results showed the system was able to accurately detect the cancer cells at a wide range of concentrations, even at very low ( <100/μl) cell densities.
One of the tests the team carried out used three different MNPs to detect cultured breast cancer cells in whole blood, and demonstrated that the system could detect multiple markers and accurately determine the level of expression of the biomarkers on each cell.
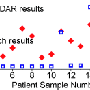
In another experiment the team used the system to detect circulating tumor cells in samples taken from 20 patients with advanced ovarian cancer, and compared the results with those obtained using CellSearch, which is known to be more accurate than flow cytometry. CellSearch detected only 18 percent of cancers in samples from stage IV patients, while the micro-Hall Detector was 100 percent accurate for these patients. Overall, CellSearch detected circulating tumor cells in 25 percent of the ovarian cancer samples, compared to 100 percent with the micro-Hall detector.
The prototype proved to be much more accurate than flow cytometry for detecting cancer cells in whole blood, especially at low numbers of tumor cells. The researchers point out that at low cancer cell numbers the rate of false positives with flow cytometry can be as high as 900% because of cell loss during the preparation of the blood sample, and because of autofluorescence arising from leukocytes in the blood. The micro-Hall Detector does not require sample purification.
The detector was also used to detect human tumor cells from xenografted mice before and after anti-cancer treatment and demonstrated that the number of tumor cells decreased after treatment.
In their paper, published in Science Translational Medicine, the researchers say the method is cost-effective and could be used in clinics to detect cancer cells. Around half a dozen biomarkers can currently be tested, but this number could increase to over 30 if the new detection system is combined with flow cytometry. The new detector could also be modified to detect pathogens as well as circulating tumor cells.
More information: Ultrasensitive Clinical Enumeration of Rare Cells ex Vivo Using a Micro-Hall Detector, Sci Transl Med 4 July 2012: Vol. 4, Issue 141, p. 141ra92. DOI: 10.1126/scitranslmed.3003747
ABSTRACT
The ability to detect rare cells (<100 cells/ml whole blood) and obtain quantitative measurements of specific biomarkers on single cells is increasingly important in basic biomedical research. Implementing such methodology for widespread use in the clinic, however, has been hampered by low cell density, small sample sizes, and requisite sample purification. To overcome these challenges, we have developed a microfluidic chip–based micro-Hall detector (μHD), which can directly measure single, immunomagnetically tagged cells in whole blood. The μHD can detect single cells even in the presence of vast numbers of blood cells and unbound reactants, and does not require any washing or purification steps. In addition, the high bandwidth and sensitivity of the semiconductor technology used in the μHD enables high-throughput screening (currently ~107 cells/min). The clinical use of the μHD chip was demonstrated by detecting circulating tumor cells in whole blood of 20 ovarian cancer patients at higher sensitivity than currently possible with clinical standards. Furthermore, the use of a panel of magnetic nanoparticles, distinguished with unique magnetization properties and bio-orthogonal chemistry, allowed simultaneous detection of the biomarkers epithelial cell adhesion molecule (EpCAM), human epidermal growth factor receptor 2 (HER2)/neu, and epidermal growth factor receptor (EGFR) on individual cells. This cost-effective, single-cell analytical technique is well suited to perform molecular and cellular diagnosis of rare cells in the clinic.
© 2012 Medical Xpress