GlaxoSmithKline pleads guilty to health fraud (Update)

(AP) — A U.S. judge on Thursday approved an agreement by British drugmaker GlaxoSmithKline to pay $3 billion for criminal and civil violations involving 10 drugs, the largest health care fraud settlement in U.S. history.
The amount of money involved led U.S. District Judge Rya Zobel to remark in court that she was having trouble keeping track of the numbers.
GlaxoSmithKline pleaded guilty to promoting the popular antidepressants Paxil and Wellbutrin for unapproved uses.
Government officials said in the original complaint that the company promoted Paxil as safe for children and adolescents, even though the U.S. Food and Drug Administration hadn't approved it for those patients and the company's clinical trials raised concerns about an increased suicide risk.
Prosecutors had charged that the drugmaker promoted Wellbutrin for unapproved uses that included treating attention deficit disorder, bipolar disorder, obesity, sexual dysfunction and anxiety when it wasn't shown to be safe and effective for those uses.
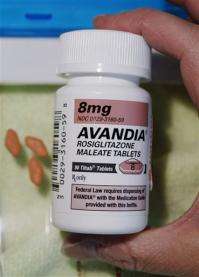
The company also admitted that it failed to report to the government some safety problems with Avandia. In 2010, the diabetes drug was restricted in the U.S. and banned in Europe after it was found to sharply increase the risks of heart attacks and congestive heart failure.
Defense lawyer Geoffrey Hobart and Assistant U.S. Attorney Sara Bloom declined to comment immediately following Thursday's hearing.
A GlaxoSmithKline spokesman referred later to comments CEO Sir Andrew Witty made Monday, including that the company has learned "from the mistakes that were made."
When the government announced the settlement Monday, U.S. Deputy Attorney General James M. Cole called it historic, saying it sent a clear warning to any company that chooses to break the law.
Copyright 2012 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.
















