Bayer receives FDA approval for long-term contraceptive
(HealthDay)—Bayer HealthCare has received approval from the U.S. Food and Drug Administration for its new low-dose levonorgestrel-releasing intrauterine system (IUS) called Skyla, according a Jan. 10 news release issued by the company.
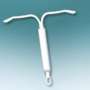
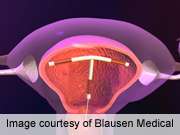
Bayer describes Skyla as a small, flexible plastic T-shaped device containing 13.5 mg of the hormone levonorgestrel. The 28 mm × 30 mm Skyla T-body is put in place through a 3.8 mm-diameter tube. A slow, low dose of levonorgestrel is locally released in the uterus, with only small amounts of the hormone entering the bloodstream. The IUS prevents pregnancy for up to three years.
The regulatory approval of Skyla was based on data from a multi-national, randomized open-label Phase 3 trial of 2,884 women (aged 18 to 35 years), in which 1,432 women received Skyla. The IUS was 99 percent effective in preventing pregnancy and was well tolerated with no unexpected adverse events. There were low incidences of ectopic pregnancy, pelvic inflammatory disease, expulsion of the device from the uterus, and uterine perforation.
"The approval of Skyla in the United States highlights Bayer's continued commitment to drive innovation and empower women with a variety of birth control options at different reproductive stages of their lives," Kemal Malik, M.D., Bayer's head of global development, said in a statement.
More information: More Information
Copyright © 2013 HealthDay. All rights reserved.