Study shows drug combination extends pancreatic cancer patient survival
A multi-center Phase III clinical trial demonstrates that Abraxane (nab-paclitaxel) plus gemcitabine is the first combination of cancer drugs to extend survival of late-stage pancreatic cancer patients compared to standard treatment.
The MPACT (Metastatic Pancreatic Adenocarcinoma Clinical Trial) study was led by physicians from Scottsdale Healthcare's Virginia G. Piper Cancer Center Clinical Trials, a partnership between Scottsdale Healthcare and the Translational Genomics Research Institute (TGen).
Their findings show that Abraxane plus gemcitabine was well tolerated and resulted in clinically meaningful outcomes compared to gemcitabine alone, the current standard of care. The study abstract was released today and the data will be presented at the American Society of Clinical Oncology (ASCO) 2013 Gastrointestinal Cancers annual meeting Jan. 25 in San Francisco.
"We are ecstatic that this clinical trial of Abraxane plus gemcitabine improves survival for patients with advanced stage IV pancreatic cancer," said Dr. Daniel Von Hoff, international lead investigator for MPACT, chief scientific officer for Virginia G. Piper Cancer Center Clinical Trials at Scottsdale Healthcare, and TGen's Physician-In-Chief. "It once again demonstrates that laboratory science based medicine can make a difference for patients."
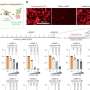
MPACT is the largest phase III clinical trial completed in advanced pancreatic cancer with more than 800 patients. Findings from the study showed a 59 percent increase in one-year median survival rates from less than a quarter of the patients (22 percent) to more than a third (35 percent). The two-year survival rate for this cancer is negligible, less than 4 percent, but that more than doubles (9 percent) with the nab-paclitaxel/gemcitabine combination.
One of those patients was Lynne Jacoby, 48, of Phoenix, who works as a director of compliance for a healthcare company. Jacoby was given only weeks to live when her Stage 4 pancreatic adenocarcinoma, a tumor the size of a golf ball, was first diagnosed in April 2012—nine months ago.
"If you had to live your life in a year, and that is all the time you have, wouldn't you do everything you could to experience this time," said Jacoby, who for nearly a year before her diagnosis had experienced night sweats, indigestion, stomach pains, neck and back pain, and an elevated white-blood count.
She began the treatment of Abraxane plus gemcitabine in May 2012 and continues on the medications, saying now that she "feels awesome, wonderful." She is scheduled to remain on the drug combination through May 2013.
"Life is priceless. No amount of money can be placed on life. I know I would be gone already if it was not for Dr. Von Hoff," said Jacoby, who also refers to him as "Dr. Von Hope."
The study showed significant improvement among some of the sickest patients including those with increased metastases. Significantly there was no increase in life-threatening toxicity. Other drug combinations that have demonstrated benefit have been limited by increased toxicities.
"This is a major improvement in a cancer with the lowest survival rates among all cancer types," said Dr. Ramesh Ramanathan, medical director of Virginia G. Piper Cancer Center Clinical Trials at Scottsdale Healthcare and principal investigator for the clinical trial in the United States. "Advanced pancreatic cancer is fourth most common cause of cancer death in the United States and throughout the world. It is difficult to diagnose with a majority of the cases diagnosed at a late stage after the disease has already advanced."
Abraxane wraps traditional chemotherapy, paclitaxel, in near-nano sized shells of albumin, a protein that the tumor sees as food. The tumor uses various mechanisms to preferentially attract the albumin, which then acts like a "Trojan Horse" to release its package of chemotherapy inside the tumor. It is approved in the U.S. for metastatic breast cancer and non-small cell lung cancer.
The pancreas is a gland behind the stomach that secretes enzymes into the upper part of the small intestine to help digestion. It also produces hormones, including insulin, which helps regulate the metabolism of sugars.
The incidence of pancreatic cancer is increasing worldwide with an estimated 279,000 cases per year, including nearly 44,000 in the U.S. in 2012, and resulting in more than 37,000 American deaths last year.