New test for skin sensitization without using animals
In an advance in efforts to reduce the use of animals in testing new cosmetic and other product ingredients for skin allergies, scientists are describing a new, highly accurate non-animal test for these skin-sensitizers. Their study appears in ACS' journal Chemical Research in Toxicology.
Bruno Miguel Neves and colleagues explain that concerns about the ethics and costs of animal-based tests for skin sensitizers, plus regulations in the European Union, are fostering a search for alternative tests. Testing product ingredients prior to marketing is important, because allergic contact dermatitis is the most prevalent form of immunotoxicity in humans.
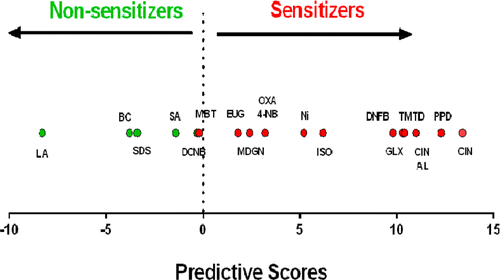
The scientists describe development of a cell-based alternative test that enlists genes and signaling pathways in mouse skin cells growing in the laboratory. Exposure to skin sensitizers triggers characteristic responses, activating genes and making cells release substances that communicate with adjacent cells. Evaluation of the test on 18 compounds showed that it had a sensitivity of 92 percent in correctly identifying actual sensitizers. It had a specificity of 100 percent and did not produce any false positive results—indicating that a substance caused sensitization when, in fact, it did not. The approach could be "extremely valuable" in revealing the interaction of skin cells with sensitizers, the scientists say.
More information: Development of an in Vitro Dendritic Cell-Based Test for Skin Sensitizer Identification, Chem. Res. Toxicol., 2013, 26 (3), pp 368–378 DOI: 10.1021/tx300472d
Abstract
The sensitizing potential of chemicals is currently assessed using animal models. However, ethical and economic concerns and the recent European legislative framework triggered intensive research efforts in the development and validation of alternative methods. Therefore, the aim of this study was to develop an in vitro predictive test based on the analysis and integration of gene expression and intracellular signaling profiles of chemical-exposed skin-derived dendritic cells. Cells were treated with four known sensitizers and two nonsensitizers, and the effects on the expression of 20 candidate genes and the activation of MAPK, PI3K/Akt, and NF-κB signaling pathways were analyzed by real-time reverse transcription polymerase chain reaction and Western blotting, respectively. Genes Trxr1, Hmox1, Nqo1, and Cxcl10 and the p38 MAPK and JNK signaling pathways were identified as good predictor variables and used to construct a dichotomous classifier. For validation of the model, 12 new chemicals were then analyzed in a blind assay, and from these, 11 were correctly classified. Considering the total of 18 compounds tested here, 17 were correctly classified, representing a concordance of 94%, with a sensitivity of 92% (12 of 13 sensitizers identified) and a specificity of 100% (5 of 5 nonsensitizers identified). Additionally, we tested the ability of our model to discriminate sensitizers from nonallergenic but immunogenic compounds such as lipopolysaccharide (LPS). LPS was correctly classified as a nonsensitizer. Overall, our results indicate that the analysis of proposed gene and signaling pathway signatures in a mouse fetal skin-derived dendritic cell line represents a valuable model to be integrated in a future in vitro test platform.